Novel method for enhancing efficiency of human T cells infected by slow-viruses carrying CAR (chimeric antigene receptor) genes
A lentivirus and cell technology, applied in the direction of retroRNA virus, virus, animal cells, etc., can solve the problems of low efficiency of CAR-T cell preparation, high cost, low efficiency of lentivirus infection of T cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Isolation and cryopreservation of human PBMC of embodiment 1
[0042] 1. Prepare experimental reagents: lymphocyte separation medium (room temperature ≈ 20°C); sterile saline (preheated to 37°C); cell freezing solution (precooled to 2-8°C).
[0043] 2. Transfer the clinically collected human peripheral blood sample into a 15ml sterile centrifuge tube, tighten the cap, adjust the centrifugal force of the centrifuge to 800g, set the centrifugal speed to the lowest, and centrifuge the blood sample at room temperature for 20 minutes.
[0044]3. Take out the centrifuge tube after centrifugation, avoid violent shaking or inverting the centrifuge tube, and transfer the upper light yellow serum layer to a new sterile centrifuge tube (after heat inactivation at 56°C for 1 hour, transfer the serum to -80°C Then add an equal volume of normal saline to the lower layer of red peripheral blood cells, tighten the cap of the centrifuge tube, and gently invert up and down to mix.
[00...
Embodiment 2
[0050] Example 2 Recovery, purification and activation of T cells
[0051] 1. Prepare T cell culture medium: 90% X-VIVO 15, 10% BI fetal bovine serum, and add IL-2, IL-15, IL-7, so that the final concentrations are 200U / ml, 5ng / ml, 5ng / ml.
[0052] 2. Take a frozen PBMC, melt in a 37°C water bath, add 10ml of rewarmed T cell culture medium, and centrifuge at 300g for 8min.
[0053] 3. Discard the supernatant, resuspend the cells with 3ml T cell culture medium, add DNaseI to make the working concentration 100μg / ml, votex, and let stand in the cell culture incubator for 15min.
[0054] 4. Filter with a 40 μm cell mesh, and count the filtered cells.
[0055] 5. Centrifuge the cell suspension at 300g for 8min.
[0056] 6. T cell purification: according to EasySep TM Instructions for the operation of the Human T Cell Isolation Kit, resuspend the cell pellet to 5×10^7 cells / ml and add corresponding volumes of Isolation Cocktail and RapidSpheres to purify CD3+ T cells.
[0057]...
Embodiment 3
[0059] Example 3 CCK-8 detects the effect of DMF on T cell proliferation
[0060] 1. Separation of T cells from Dynabeads: Transfer T cells activated for 72 hours to a 15ml centrifuge tube, and mix well by pipetting. Place the centrifuge tube in the EasySep TM Magnetic pole, stand at room temperature for 3 minutes, transfer the cell suspension to a new 15ml centrifuge tube, and count.
[0061] 2. Inoculate T cells in a 96-well plate at 1×10^5 cells / 100 μl / well, and treat them with different concentrations of DMF (0, 25 μM, 50 μM, 100 μM, 150 μM, 200 μM), and set 3 replicate wells for each concentration , continue to cultivate for 22 hours.
[0062] 3. Add 10 μl of CCK-8 to each well, and mix well by pipetting. The culture plate was incubated in the incubator for 1-4 hours, and the absorbance at 450 nm was measured with a microplate reader.
[0063] 4. Calculate the inhibition rate at different concentrations.
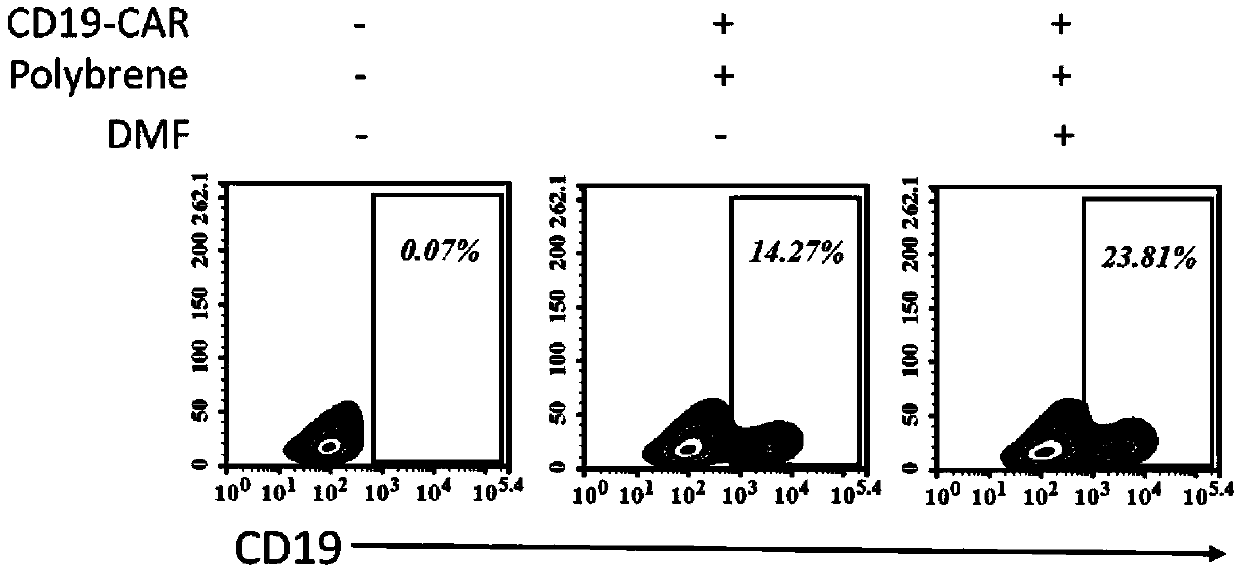
[0064] The result is as figure 1 As shown, the treatment und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com