Polyarylene ether/polyarylene sulfide with thermally activated delayed fluorescence effect and its preparation method and application
A thermal activation delay, polyarylene sulfide technology, applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, luminescent materials, etc., can solve problems such as unfavorable device performance, low skeleton triplet energy level, affecting luminous efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0198] Embodiment 1: the synthesis of polymer Ⅰ-1
[0199] (1) Material number m-1
[0200] The reaction equation is as follows:
[0201]
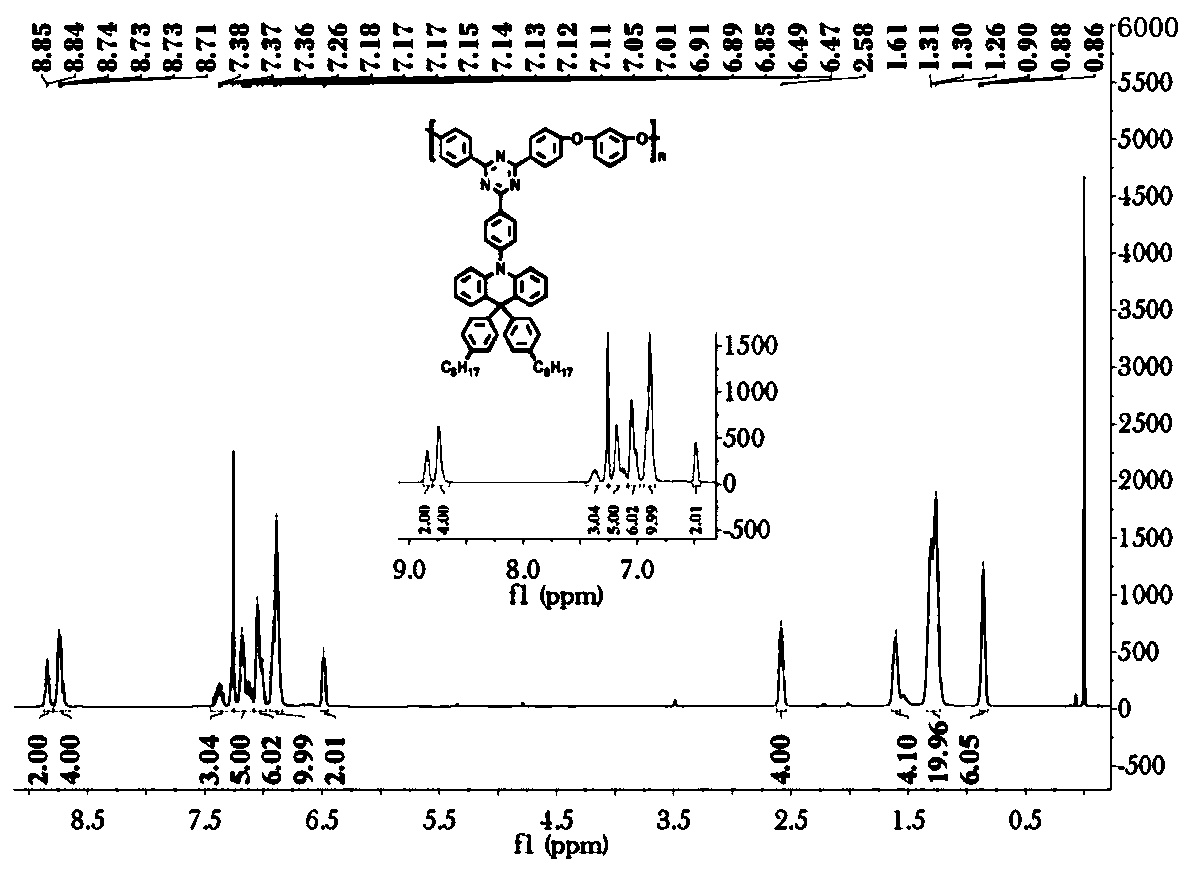
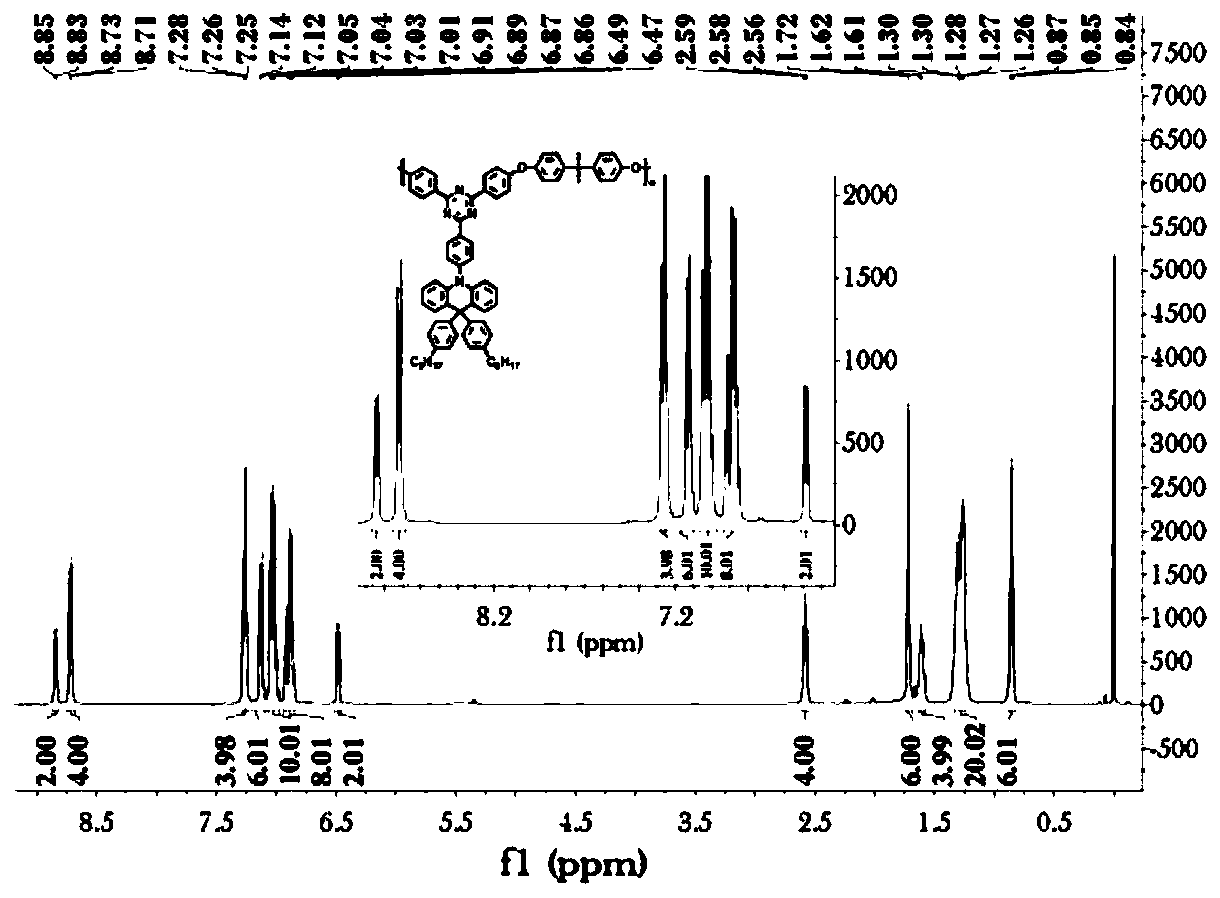
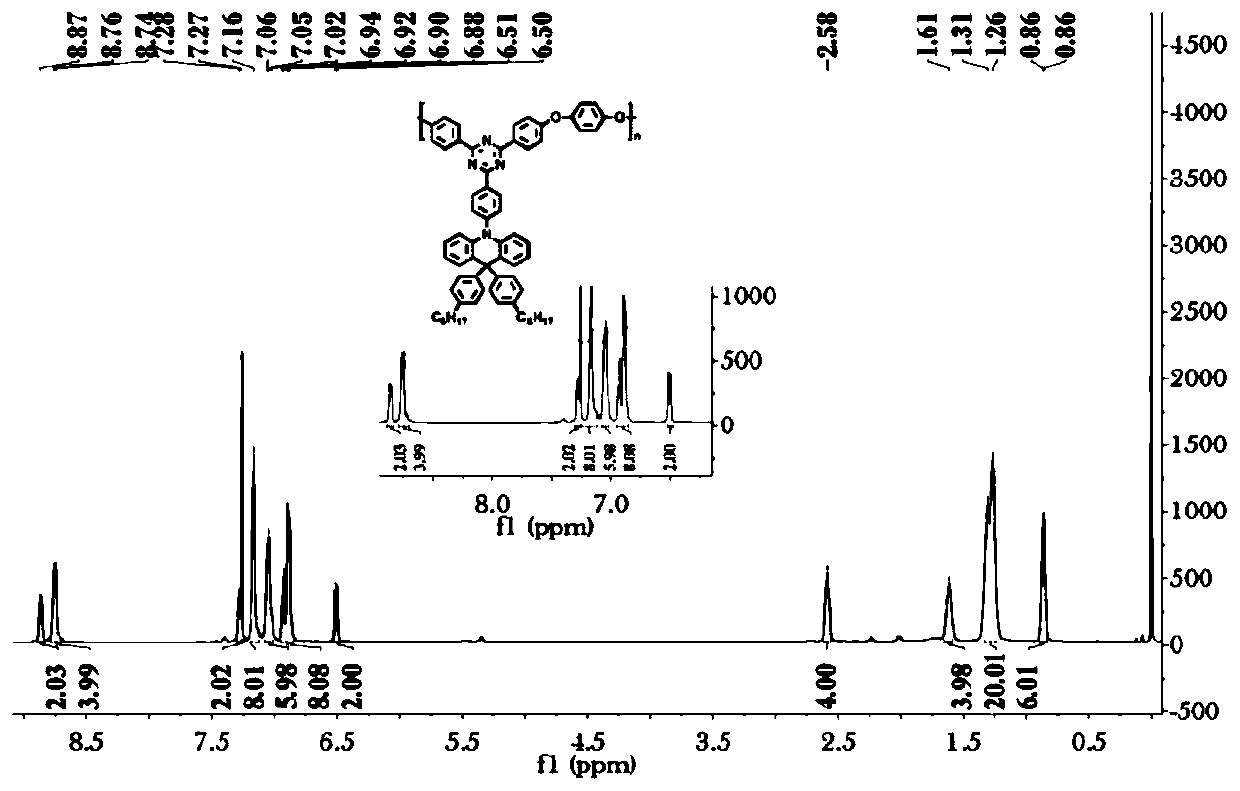
[0202]The specific steps are: in a 1000ml three-neck round bottom flask, N-phenylanthranilic acid (108.61g, 0.5mol) was dissolved in methanol (300ml). Thionyl chloride (76ml) was carefully added dropwise. React at 60°C for 12h. After the reaction is completed, cool down to room temperature, add saturated aqueous sodium bicarbonate solution, extract the organic phase with dichloromethane, dry over anhydrous sodium sulfate, remove the solvent by rotary evaporation, and use a mixed solvent of petroleum ether:dichloromethane=5:1 as the eluent , and separated by silica gel column chromatography to obtain 110 g of yellow solid with a yield of 95%. 1 H NMR (400MHz, DMSO, δppm): 9.32(s, 1H), 7.90(d, J=8.0Hz, 1H), 7.44–7.33(m, 3H), 7.24(t, J=7.7Hz, 3H), 7.09 (t, J = 7.2Hz, 1H), 6.81 (t, J = 7.5Hz, 1H), 3.86 (s, 3H).
[0203] (2) Material n...
Embodiment 2
[0228] Embodiment 2: the synthesis of polymer I-2
[0229] The reaction equation is as follows:
[0230] The specific steps are: add m-6 (0.2g, 0.22mmol), resorcinol (0.0242g, 0.22mmol) and potassium carbonate (0.0730g, 0.53mmol) into a Schlenk bottle with an oil-water separator and a spherical condenser After adding 1ml N,N-dimethylacetamide and 1.5ml toluene, heated and stirred at 120°C for 3h under argon atmosphere, then raised the temperature to 150°C, reacted for 2h, and finally raised the temperature to 170°C, reacted for 24h. After the reaction, the reaction solution was poured into chloroform, the organic phase was washed three times with saturated brine and then dried with anhydrous sodium sulfate. After the organic phase was concentrated, it was settled in n-hexane, and the obtained yellow powdery solid was extracted with acetone for 24 hours. , extracted with methanol for 24 hours, and dried in vacuo to obtain 0.14 g of the product with a yield of 64%.
[0231] ...
Embodiment 3
[0234] Embodiment 3: the synthesis of polymer I-3
[0235] The reaction equation is as follows:
[0236]
[0237] The specific steps are: add m-6 (0.2g, 0.22mmol), hydroquinone (0.0242g, 0.22mmol) and potassium carbonate (0.0730g, 0.53mmol) into a Schlenk bottle with an oil-water separator and a spherical condenser After adding 1ml N,N-dimethylacetamide and 1.5ml toluene, heated and stirred at 120°C for 3h under argon atmosphere, then raised the temperature to 150°C, reacted for 2h, and finally raised the temperature to 170°C, reacted for 24h. After the reaction, the reaction solution was poured into chloroform, the organic phase was washed three times with saturated brine and then dried with anhydrous sodium sulfate. After the organic phase was concentrated, it was settled in n-hexane, and the obtained yellow powdery solid was extracted with acetone for 24 hours. , extracted with methanol for 24 hours, and dried in vacuo to obtain 0.16 g of the product with a yield of 73%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com