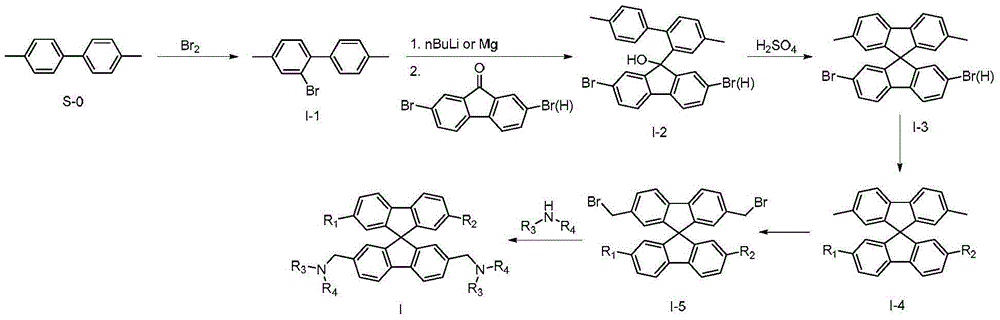
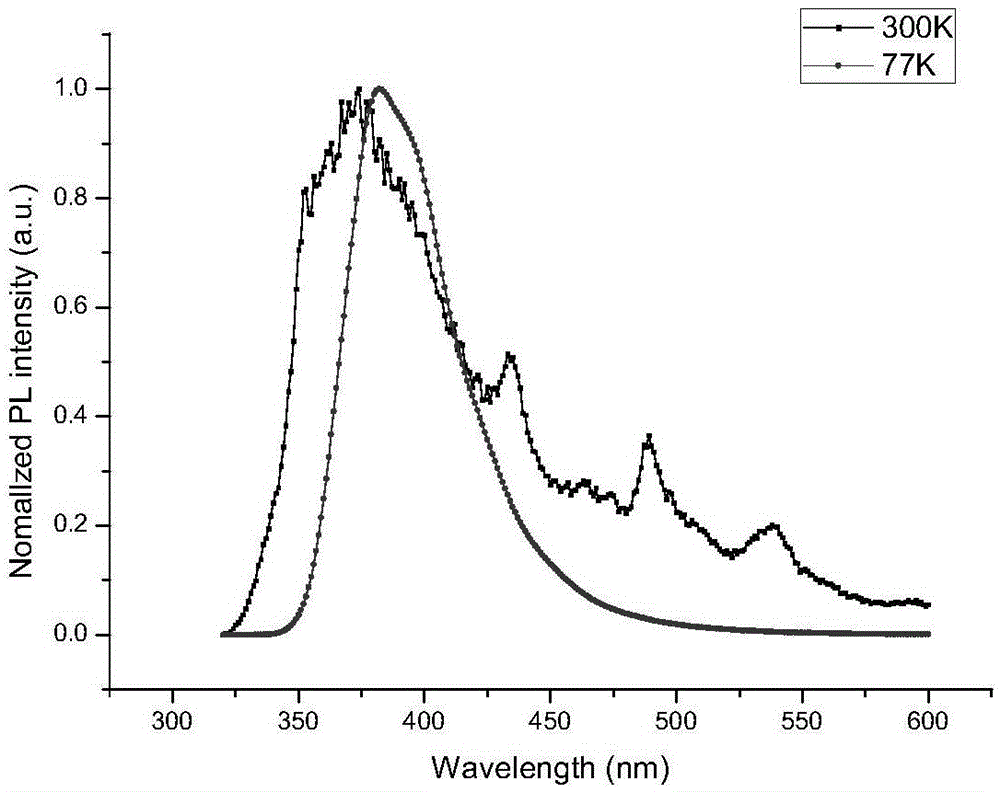
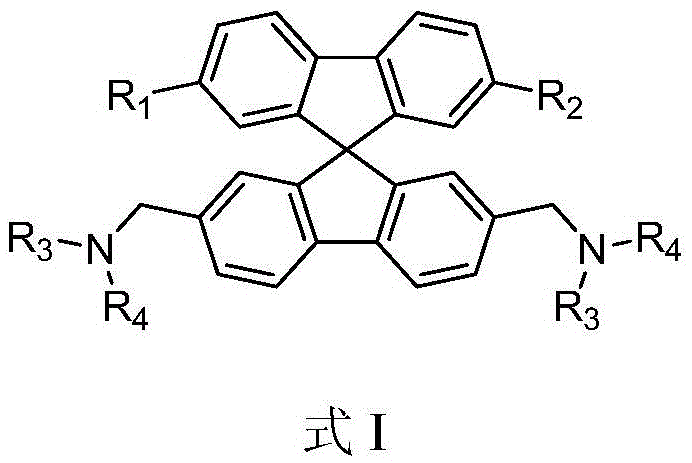
Spirofluorene benzyl fluorescent material
A technology based on phenyl and aryl groups, applied in the field of organic electroluminescence display, can solve the problems of difficulty in improving luminous efficiency and small luminous contribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The preparation of embodiment 1 compound I001
[0080]
[0081] The first step: the preparation of 2-bromo-4,4'-dimethylbiphenyl (I-1)
[0082]
[0083] Dissolve 36.5g (0.2mol) of 4,4'-dimethylbiphenyl in 360ml of anhydrous dichloromethane, add 1 grain of iodine at room temperature, stir for 1 hour, slowly add 35.2g (0.22mol) of bromine dropwise The solution dissolved in dichloromethane was stirred and reacted for 24 hours, 120ml of saturated aqueous sodium bisulfite solution was added, stirred and reacted for 1 hour, the organic phase was separated, the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure , separated and purified with a silica gel column, and concentrated to dryness under reduced pressure to obtain 48 g of I-1 as a colorless oil, with a yield of 92%.
[0084] The second step: the preparation of intermediate I-2
[0085]
[0086] Stir and dissolve 20g of intermedi...
Embodiment 2
[0105] The preparation of embodiment 2 compound I017
[0106]
[0107] The first step: the preparation of intermediate I-4
[0108]
[0109] Under the protection of nitrogen, 10.4g of the intermediate I-3 prepared in Example 1 was dissolved in 120ml of dry tetrahydrofuran, placed in a low-temperature tank and cooled to -78°C, and 20ml of 2.5M n-butyllithium-hexane solution was added dropwise , stirred and reacted for 30 minutes, added dropwise a solution of 9.2g of N,N-diethylbenzamide dissolved in tetrahydrofuran, stirred and reacted for 30 minutes, raised to room temperature and stirred for 1 hour, added 100ml of 3M dilute hydrochloric acid aqueous solution, stirred and reacted 1 hour. Extract three times with ethyl acetate. The organic phases were combined and washed with 100ml of saturated brine, the organic phase was dried with anhydrous sodium sulfate, filtered, the filtrate was concentrated to dryness under reduced pressure, the residue was separated and purifie...
Embodiment 3
[0122] The preparation of embodiment 3 compound I051
[0123]
[0124] The first step: the preparation of intermediate I-4
[0125]
[0126] Under nitrogen protection, 10.0g of the intermediate I-3 prepared in Example 1 was dissolved in 120ml of dry N,N-dimethylformamide, 5.5g of thiophenol and 13.0g of anhydrous cesium carbonate were added and 38mg of cuprous iodide, stirred and reacted for 30 minutes, then added 0.5ml of N,N'-dimethylethylenediamine, heated to 100°C, stirred and reacted for 12 hours, cooled to room temperature, poured the reaction solution into 500ml of dilute In aqueous hydrochloric acid solution, stirred and reacted for 1 hour, filtered, washed the filter cake with water, and recrystallized with dichloromethane-ethanol to obtain 9.3 g of an intermediate, a yellow solid, with a yield of 84%.
[0127] The above intermediate of 9.3g was dissolved in 150ml of dichloromethane, 19g of m-chloroperoxybenzoic acid (75%) was added in batches, stirred and reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com