Chiral fluorescent probe with spiropyran characteristics as well as preparation method and application thereof
A technique of fluorescent probe and spiropyran, which is applied in the field of fluorescent probes and achieves broad application prospects and simple synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 chiral fluorescent probe
[0043] A preparation method of a chiral fluorescent probe with spiropyran characteristics, comprising the following steps:
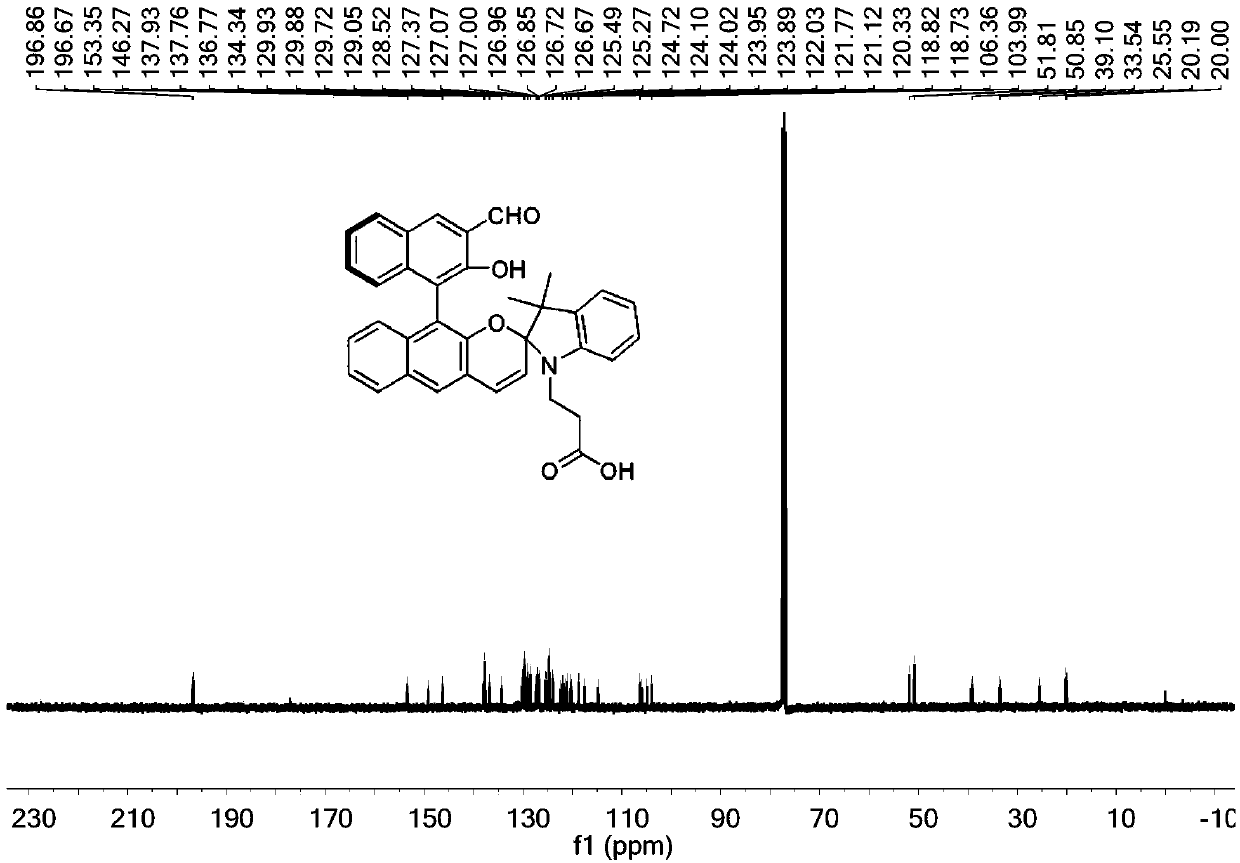
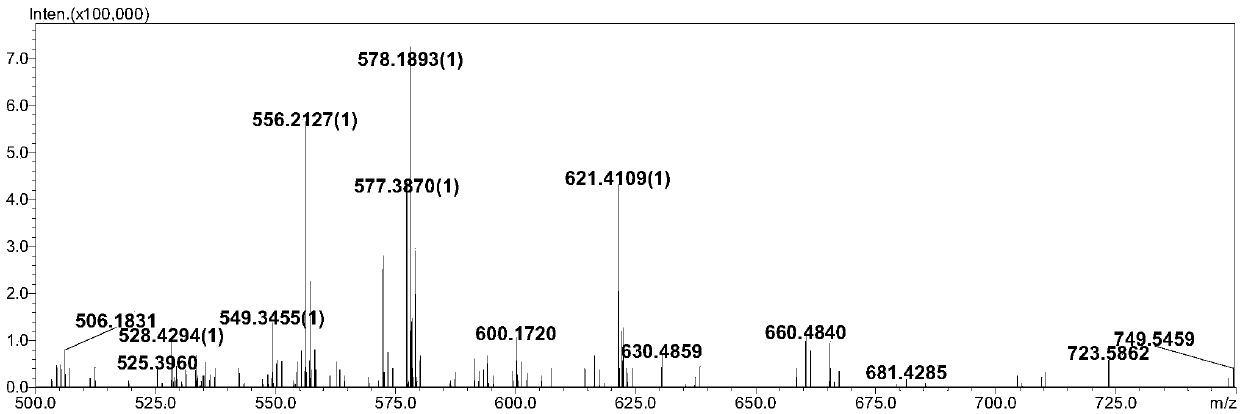
[0044] 1-propionic acid-2,3,3-trimethylindole quaternary ammonium salt (377mg, 0.8mmol), (S)-2,2'-dihydroxy-1,1'-binaphthyl-3,3 '-Dialdehyde group (450mg, 1.32mmol) and piperidine (134mg, 1.58mmol) were added to 15mL ethanol solution, and heated to reflux for 6h. After the reaction, the product was distilled to remove the solvent, and then dichloromethane and methanol The volume ratio is 40:1 and mixed as eluent, and purified by column chromatography to obtain the target product, which is designated as probe (S)-3. 1 HNMR spectrum as figure 1 as shown, 13 C NMR spectrum as figure 2 As shown, the mass spectrum is as image 3 shown.
[0045] The preparation process of probe (R)-3 is the same as the preparation process of probe (S)-3, the only difference is that the starting material i...
Embodiment 2
[0046] Embodiment 2 Identification of chiral molecules
[0047] 1. Recognition of Phenylalanine
[0048] Dissolve the fluorescent probes (S)-3 and (R)-3 prepared in Example 1 in tetrahydrofuran solution to make the concentration 2mM, then draw 20μL into a glass test tube, add 50mM HEPES buffer to dilute to 2mL (diluted probe concentration is 2×10 -5 mol / L; H 2 O / THF=99 / 1, THF is 1%, and the system is 1% THF / HEPES system), then add 2 equivalents of zinc acetate and different equivalents of phenylalanine, measure the change of fluorescence intensity after 2h, the results are shown in Figure 4-7 . Depend on Figure 4-7 It can be seen that the chiral fluorescent probe probe (S)-3 has a large difference in fluorescence intensity for different configurations of phenylalanine, and has good enantioselectivity, with an ef value of about 9.6.
[0049]2. Recognition of leucine, valine, tryptophan and methionine
[0050] According to the above-mentioned identification method for ph...
Embodiment 3
[0052] Example 3 Cell Imaging of Phenylalanine Chiral Molecules
[0053] The fluorescent probe (S)-3 of Example 1 was applied to HeLa cells for fluorescence imaging, and the specific operation steps were as follows:
[0054] (1) The density of 3 parts is 3×10 5 cells / mL of HeLa cells at a temperature of 37°C, CO 2 Culture in an incubator with a concentration of 5% until the cells adhere to the wall;
[0055] (2) Take an aliquot of cells, add 20 μM (S)-3 and incubate for 30 min, wash the cells with PBS buffer 3 times, image under a fluorescent microscope after sample preparation, and the excitation wavelength is 445 nm;
[0056] (3) Take a portion of the cells, add 20 μM (S)-3 and incubate for 30 minutes, add D-phenylalanine and incubate for 2 hours, wash the cells with PBS buffer for 3 times, image under a fluorescent microscope after sample preparation, and the excitation wavelength is 445nm ;
[0057] (3) Take a portion of the cells, add 20 μM (S)-3 and incubate for 30 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com