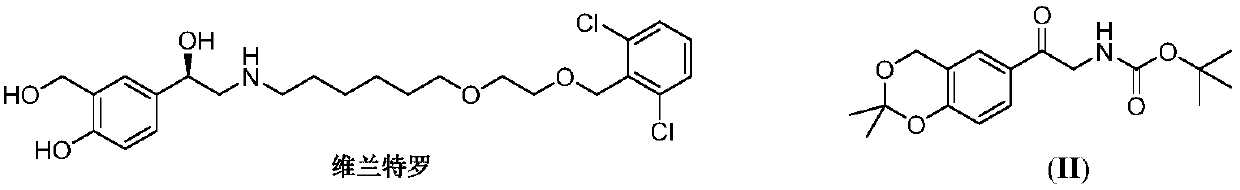
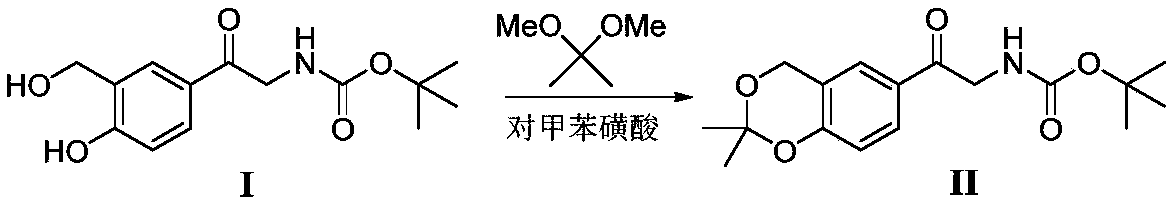
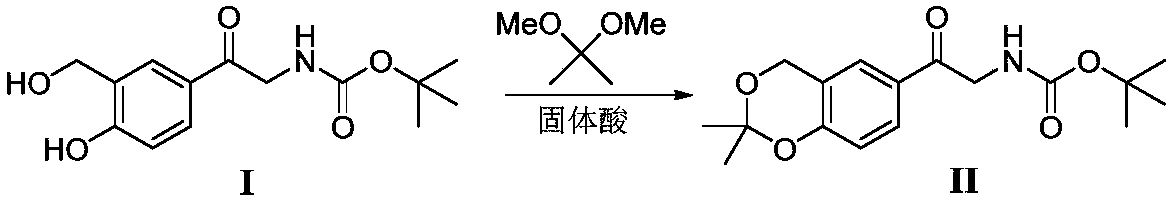
Method for synthesizing Vilanterol midbody by virtue of solid acid catalysis
A technology of solid acid catalysis and intermediates, applied in chemical recycling, organic chemistry, etc., can solve the problems of low yield, poor color, deep product color, etc., achieve simple post-processing, reduce environmental protection pressure, and increase productivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 28.1g intermediate (I), 2,2-dimethoxypropane (20.8g), SO 4 2- / ZrO 2 (1.4g), add chloroform (140mL), heat up to 60°C, react for 2 hours, and the reaction is completed in TLC. Cool down to room temperature, filter, and concentrate the filtrate in vacuo to obtain a crude product, which is recrystallized from isopropyl ether to obtain 29.2 g of a white solid with a yield of 91% and a purity of 99%.
Embodiment 2
[0022] Add 28.1g intermediate (I), 2,2-dimethoxypropane (20.8g), SO 4 2- / TiO 2 (1.4g), add 1,2-dichloroethane (140mL), heat up to 55°C, react for 3 hours, and the reaction is completed in TLC. Cool down to room temperature, filter, and concentrate the filtrate in vacuo to obtain a crude product, which is recrystallized from isopropyl ether to obtain 29.5 g of a white solid with a yield of 92% and a purity of 99%.
Embodiment 3
[0024] Add 28.1g intermediate (I), 2,2-dimethoxypropane (20.8g), PO 4 3- / TiO 2 (1.4g), add acetone (140mL), heat up to 50°C, react for 3 hours, and the reaction is completed in TLC. Cool down to room temperature, filter, and concentrate the filtrate in vacuo to obtain a crude product, which is recrystallized from isopropyl ether to obtain 29.2 g of a white solid with a yield of 91% and a purity of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com