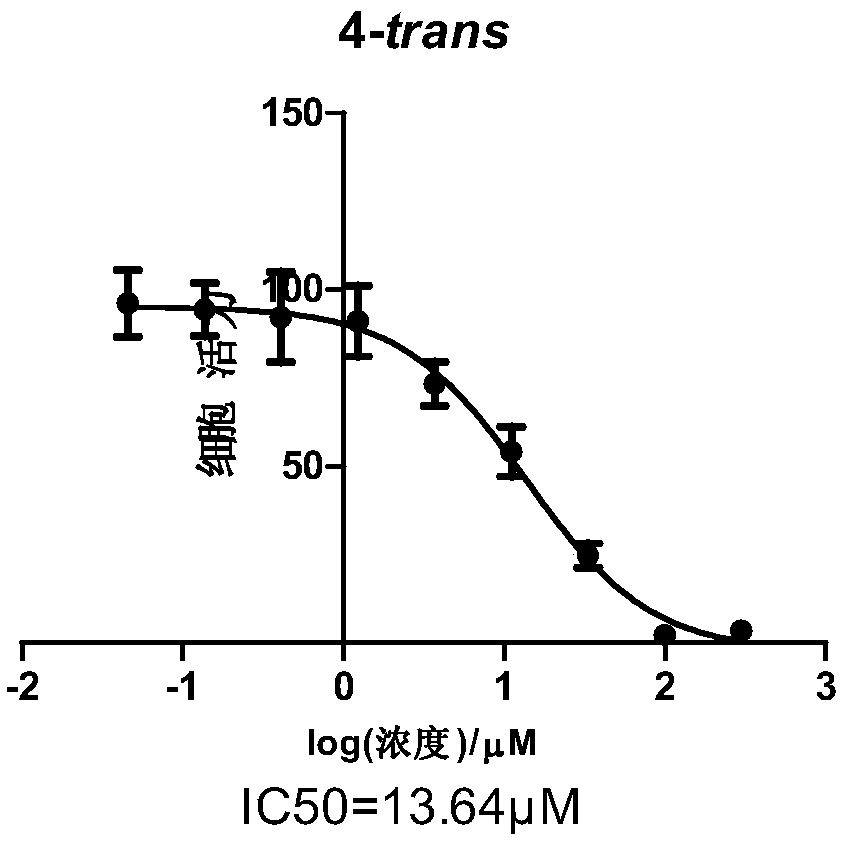
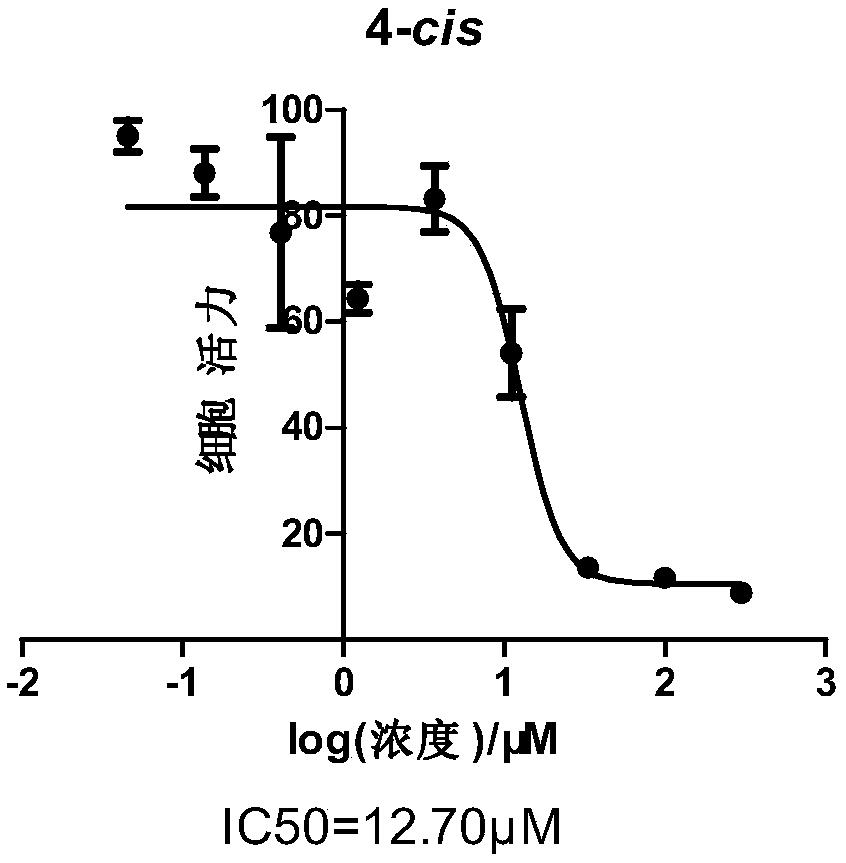
Acetamino azobenzene derivative and preparation and application thereof
A technology for acetamidoazobenzenes and derivatives is applied in the fields of acetamidoazobenzene derivatives and their preparation and application, can solve problems such as lack of availability, and achieve the effects of easy separation and purification, and a simple and convenient preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation process of (E)-2-chloro-N-(4-((4-chlorophenyl) azo) phenyl) acetamide is as follows:
[0038]
[0039] Reagents and conditions: (a) K 2 CO 3 ,H 2O, 10min; (b) AcOH, DCM, r.t. overnight (c) 1) HCl, MeOH, reflux, 9h; 2) NaOH; (d) chloroacetyl chloride, dry DCM, DIPEA, r.t., overnight.
[0040] Specifically, the following steps are included:
[0041] (1), intermediate 1[N-(4-nitrosophenyl)acetamide]
[0042]
[0043] Dissolve potassium hydrogen persulfate compound salt in 50ml of water, add potassium carbonate to adjust the pH to weak acidity, then quickly add 4-aminoacetanilide that has been dissolved in water, the solution quickly produces gray-green foam and gray-green precipitation, and when the gray-green After the foam disappeared and the precipitate settled, the reaction was continued for another 10 minutes to stop. Filtrate to obtain a gray-green precipitate, dissolve it with hot ethanol and filter to remove impurities to obtain a green s...
Embodiment 2
[0054] (E)-2-chloro-N-(4-((2,4-dichlorophenyl) azo) phenyl) acetamide preparation process is as follows:
[0055]
[0056] Reagents and conditions: (a) K 2 CO 3 ,H 2 O, 30min; (b) AcOH, DCM, r.t.overnight (c) 1) HCl, MeOH, reflux, 9h; 2) NaOH; (d) chloroacetyl chloride, dry DCM, DIPEA, r.t., overnight.
[0057] Specifically, the following steps are included:
[0058] (1), intermediate 5[2,4-dichloro-1-nitrosobenzene]
[0059]
[0060] Dissolve 2,4-dichloroaniline in dichloromethane, dissolve potassium hydrogen persulfate complex salt in water, mix under nitrogen protection, and stir overnight at room temperature. After the reaction, the organic phase was washed with 1M hydrochloric acid (10 mL) and saturated brine. Finally, dry the organic phase with anhydrous sodium sulfate, concentrate the product intermediate 5, and directly put it into the next step of reaction.
[0061] (2), intermediate 6[(E)-N-(4-((2,4-dichlorophenyl)azo)phenyl)acetamide]
[0062]
[006...
Embodiment 3
[0071] Preparation of (E)-2-chloro-N-(4-((3,4-dichloro)azo)phenyl)acetamide (9)
[0072] Adopt the synthetic method similar to embodiment 2, difference is:
[0073] (a), the raw material amine described in step 1 adopts 3,4-dichloroaniline
[0074] The final test results are as follows: 1H NMR (400MHz, DMSO-d6): δ10.71(s, 1H), 8.02(s, 1H), 7.92(d, J=7.6Hz, 2H), 7.85(s, 1H), 7.84(s,1H),7.82(d,J=9.2Hz,2H),4.32(s,2H) ppm.HRMS(ESI):[M+H] – C 14 h 11 Cl 3 N 3 O, the target compound Exact mass calculated value: 341.9959, observed value: 341.9967.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com