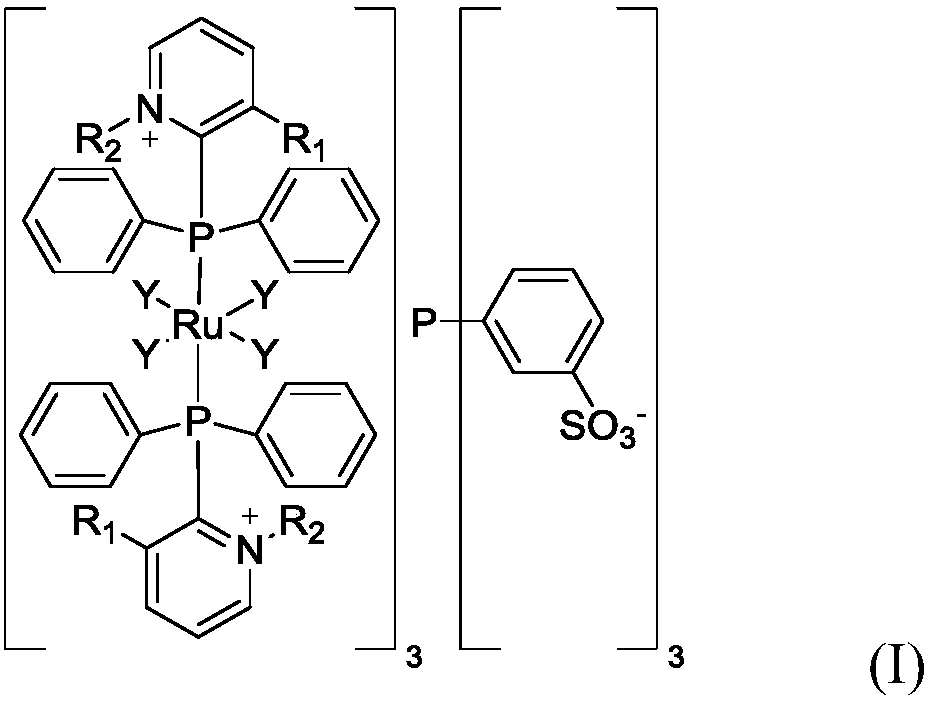
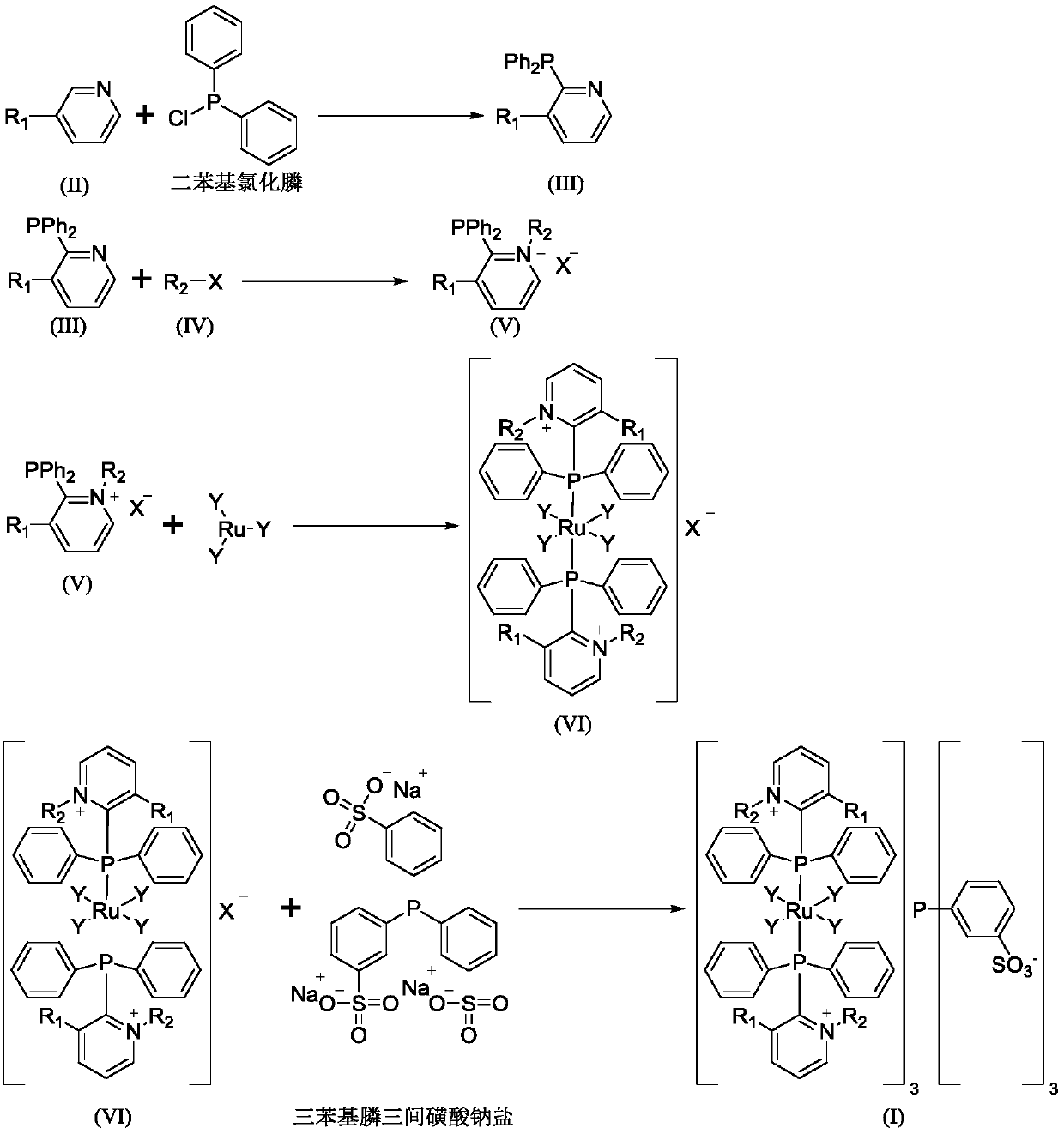
Phosphine-ruthenium functionalized ionic liquid and preparation method thereof, catalyst and preparation method for 4-acetoxybutyraldehyde
An ionic liquid and acetoxy technology, which is applied in the preparation of organic compounds, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problems of easy hydrolysis, weak coordination, and easy catalyst Inactivation and other problems, to achieve high conversion rate, high reaction conversion rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] To prepare phosphine-ruthenium functionalized ionic liquid IL1, proceed as follows:
[0058] (1), under nitrogen protection, 3-picoline (13.97g, 0.15mol) was dissolved in tetrahydrofuran (540.83g, 7.5mol), and n-butyllithium hexane solution was added dropwise at -70°C ( 2M, 90mL) for 5 hours, followed by adding diphenylphosphine chloride (39.72g, 0.18mol) for 1.5 hours, and then warming up to room temperature for 12 hours. After the reaction, it was quenched with ammonium chloride. Spin dry under reduced pressure under the protection of nitrogen, and recrystallize with dichloromethane / methanol solution with a volume ratio of 1:10 to obtain a white solid Its nuclear magnetic analysis data are as follows: 1 H NMR (400MHz, CDCl 3 ,298K)δ=8.7(1H,dd,J=8.6Hz,2.4Hz, N CH =CH),8.0(1H,dd,J=8.6Hz,2.4Hz,CH 3 -C CH =CH), 7.2-7.5(11H, m, NCH= CH , Phenyl -P),2.3(3H,s, CH 3 ).
[0059] (2), (37.44g, 0.135mol) the product of step (1) was mixed with bromomethane tetrahydro...
Embodiment 2
[0066] Preparation of phosphine-ruthenium functionalized ionic liquid IL2 is carried out according to the following steps:
[0067] (1) Under nitrogen protection, dissolve 3-phenylpyridine (15.52g, 0.1mol) in tetrahydrofuran (1081.65g, 15mol), and add n-butyllithium hexane solution (2M , 100mL) to keep warm for 3h, then add diphenylphosphine chloride (44.13g, 0.2mol) to keep warm for 1h, then warm up to room temperature and react for 10h, after the reaction, quench with ammonium chloride, and the reaction solution is protected under nitrogen Spin dry under reduced pressure, and recrystallize with a dichloromethane methanol solution with a volume ratio of 10:1 to obtain a white solid Its nuclear magnetic analysis data are as follows: 1 H NMR (400MHz, CDCl 3 ,298K)δ=8.7(1H,dd,J=8.6Hz,2.5Hz, N CH =CH), 8.3(1H, dd, J=8.6Hz, 2.5Hz, Phenyl-C CH =CH),7.8(1H,t,J=8.6Hz, NCH CH =CH),7.3-7.5(15H,m, Phenyl group ).
[0068] (2), (31.22g, 0.09mol) step (1) product is mixed with mo...
Embodiment 3
[0074] Preparation of phosphine-ruthenium functionalized ionic liquid IL3 is carried out according to the following steps:
[0075] (1), under the protection of nitrogen, dissolve 3-picoline (9.31g, 0.1mol) in tetrahydrofuran (721.10g, 10mol), and add n-butyllithium hexane solution (2M , 80mL) to keep warm for 4h, then add diphenylphosphine chloride (35.30g, 0.16mol) to keep warm for 1h, then warm up to room temperature and react for 9h, after the reaction, quench with ammonium chloride, and the reaction solution is protected under nitrogen Spin dry under reduced pressure, and recrystallize with a dichloromethane / methanol solution with a volume ratio of 1:1 to obtain a white solid Its nuclear magnetic analysis data are as follows: 1 H NMR (400MHz, CDCl 3 ,298K)δ=8.7(1H,dd,J=8.6Hz,2.4Hz,N CH =CH),8.0(1H,dd,J=8.6Hz,2.4Hz,CH 3 -C CH =CH), 7.2-7.5(11H, m, NCH= CH , Phenyl -P),2.3(3H, s, CH 3 ).
[0076] (2), (25.79g, 0.09mol) the product of step (1) is mixed with mono...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com