Method for constructing bisindole substituted dihydropyrrole derivative based on oxime ester and indole
A technology of dihydropyrrolidone and derivatives, applied in the direction of organic chemistry, etc., can solve the problems of harsh conditions, many reaction steps, difficult post-processing, etc., and achieves the effects of easy fracture, wide applicability, and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
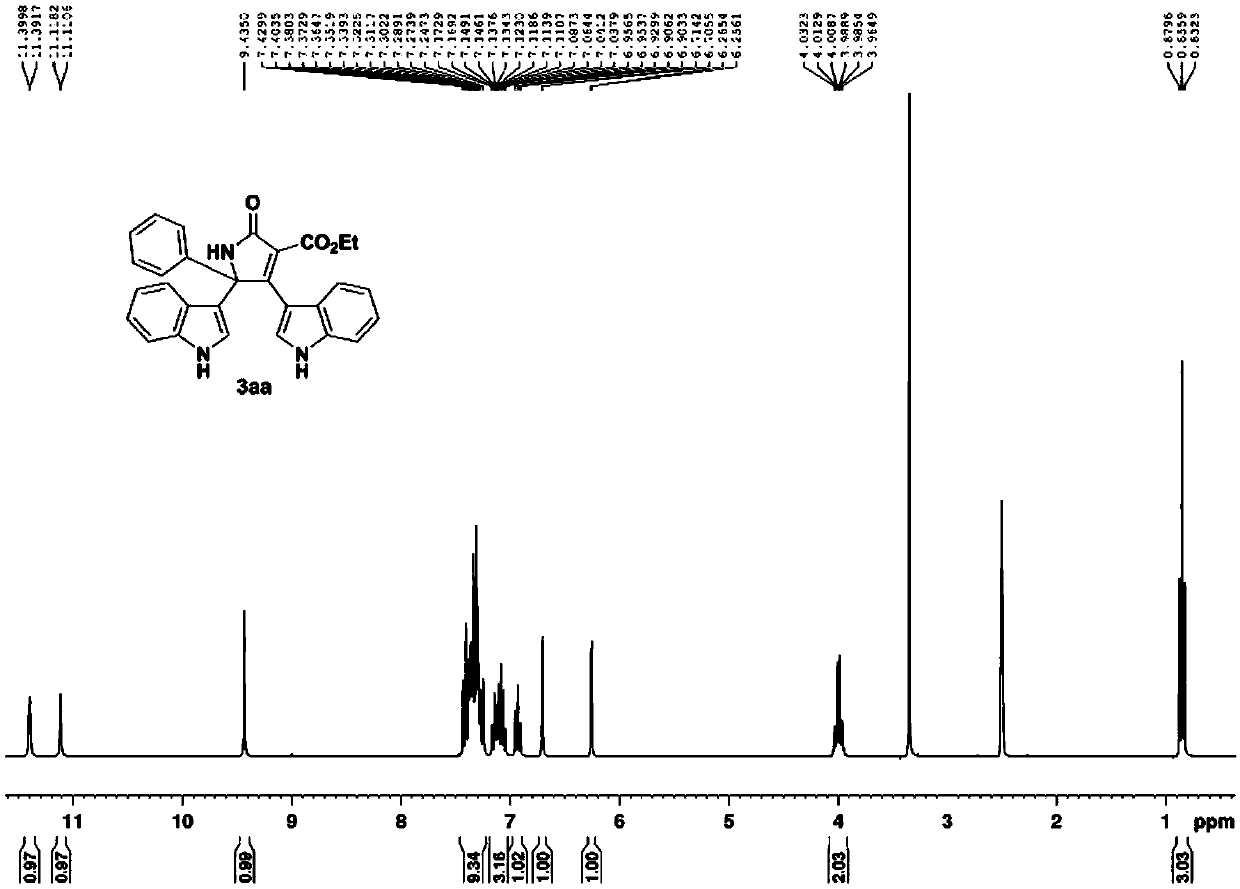
[0051] Acetophenone oxime acetate substituted with indole and diethyl malonate is raw material, according to indole: oxime acetate: copper acetate molar ratio is 1.6:1:2, oxime acetate 0.2mmol, solvent The amount of acetonitrile is 4mL, in N 2 Heated to 80°C under protective conditions, and TLC detected that the reaction was progressing. After all the raw materials were completely reacted, they were extracted, spin-dried, and dissolved in THF to dissolve the final product 3aa washed with ethyl acetate, with a yield of 85%.
[0052]
[0053] The obtained product is tested, and the instruments used for the test are: AVANCE 300MHz nuclear magnetic resonance instrument (Bruker company, TMS is the internal standard); SGW X-4 micro melting point instrument (the thermometer is not calibrated). The test method of the following examples is the same as that of this example.
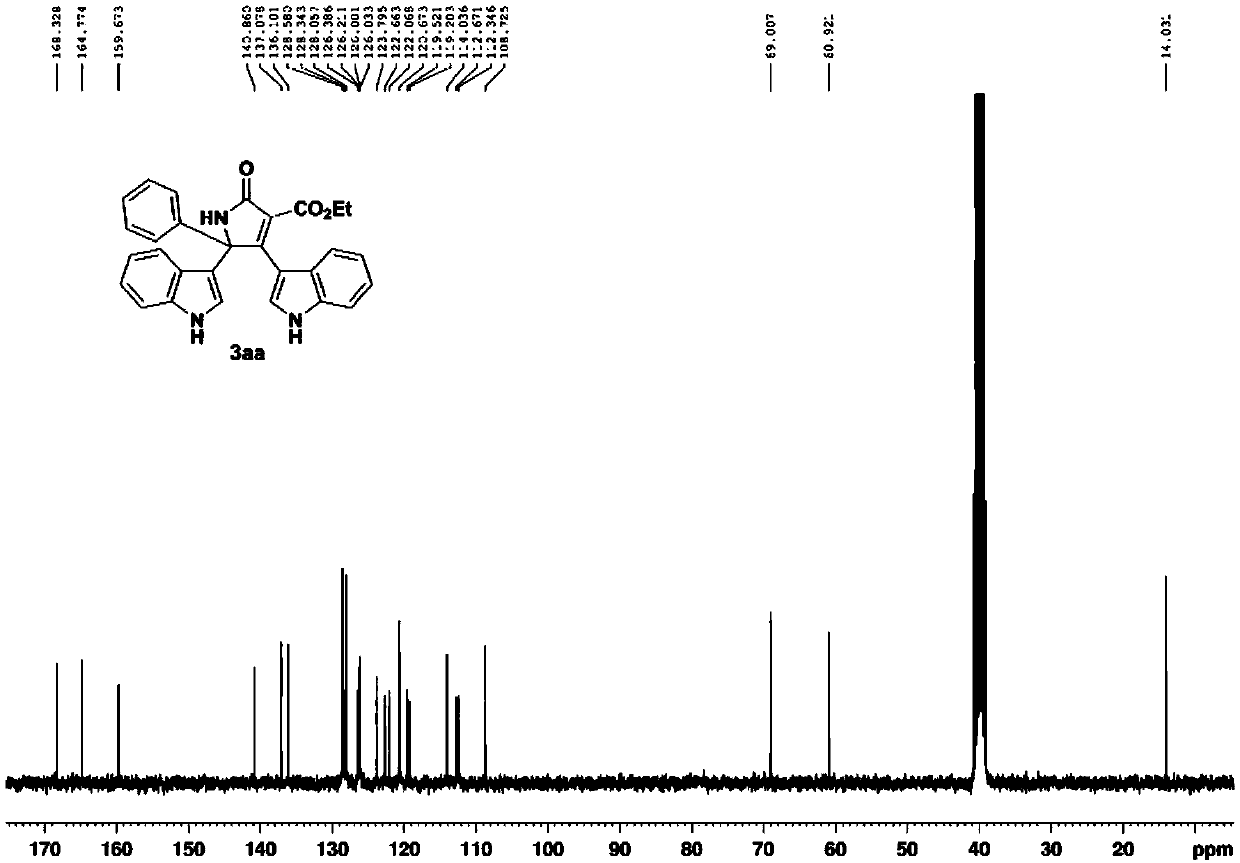
[0054] 3aa: brown powdery solid, mp 295.4-296.7℃. 1 H NMR (300MHz, d 6 -DMSO) δ11.60(d, J=2.4Hz, 1H), 11.1...
Embodiment 2
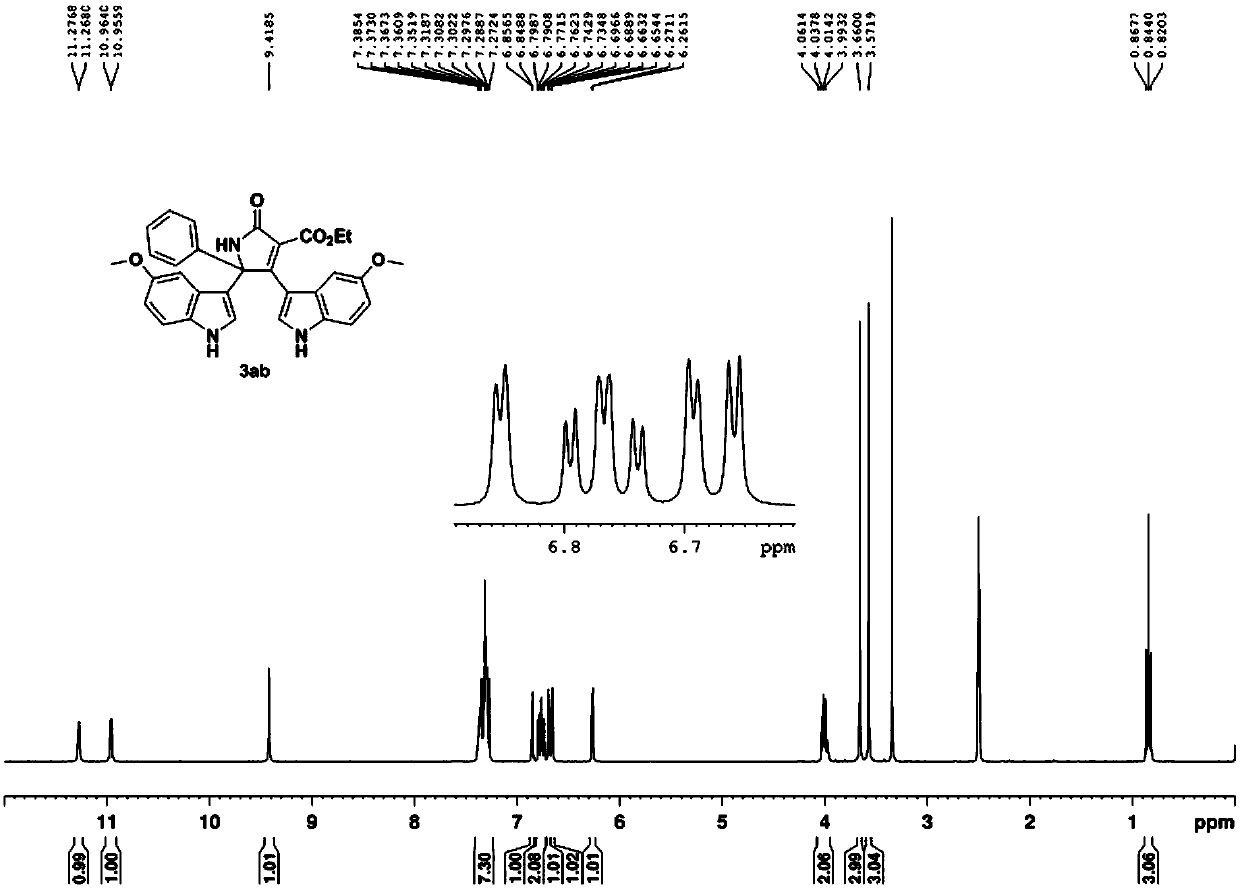
[0056] With 5-methoxyindole, the acetophenone oxime acetate substituted by diethyl malonate as raw material, according to 5-methoxyindole: oxime acetate: copper acetate molar ratio is 1.6:1: 2, Oxime acetate 0.2mmol, the amount of solvent acetonitrile is 4mL, in N 2 Heated to 80°C under protective conditions, and TLC detected that the reaction was progressing. After all the raw materials were completely reacted, they were extracted with ethyl acetate and water, dried and spin-dried, and recrystallized from 3 mL of ethyl acetate to obtain the pure product 3ab with a yield of 78%.
[0057]
[0058] 3ab: yellow solid, mp 220-223℃. 1 H NMR (300MHz, d 6 -DMSO)δ11.27(d,J=2.6Hz,1H),10.96(d,J=2.4Hz,1H),9.42(s,1H),7.27-7.38(m,7H),6.85(d,J =2.3Hz,1H),6.77(td,J=8.4Hz,2.5Hz,2H),6.69(d,J=2.3Hz,1H),6.66(d,J=2.6Hz,1H),6.27(d, J=2.9Hz, 1H), 4.00(q, J=7.1Hz, 2H), 3.66(s, 3H), 3.57(s, 3H), 0.84(t, J=7.1Hz, 3H); 13 C NMR (75MHz, d 6 -DMSO) δ168.4, 165.1, 159.6, 154.7, 153.5, 140.7, 132.1...
Embodiment 3
[0060] With 7-methylindole, the acetophenone oxime acetate substituted by diethyl malonate as raw material, according to 7-methylindole: oxime acetate: copper acetate mol ratio is 1.6:1:2, Oxime acetate 0.2mmol, solvent acetonitrile consumption is 4mL, in N 2 Heated to 80°C under protective conditions, and TLC detected that the reaction was progressing. After all the raw materials were completely reacted, they were extracted with ethyl acetate and water, dried and spin-dried, dissolved in methanol, and washed with ethyl acetate to obtain the pure product 3ac with a yield of 82%.
[0061]
[0062] 3ac: Yellow-green solid, mp 203.4-204.2℃. 1 H NMR (300MHz, d 6 -DMSO) δ11.39(d, J=2.6Hz, 1H), 11.08(d, J=2.3Hz, 1H), 9.43(s, 1H), 7.27-7.40(m, 5H), 7.11-7.20(m ,2H),6.81-7.02(m,4H),6.65(d,J=2.6Hz,1H),6.16(d,J=2.9Hz,1H),4.00(q,J=7.1Hz,2H),2.44 (s,3H),2.40(s,3H),0.89(t,J=7.1Hz,3H); 13 C NMR (75MHz, d 6 -DMSO) δ168.4, 164.8, 159.8, 140.8, 136.5, 135.6, 128.6, 128.4, 128.2, 125.9,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com