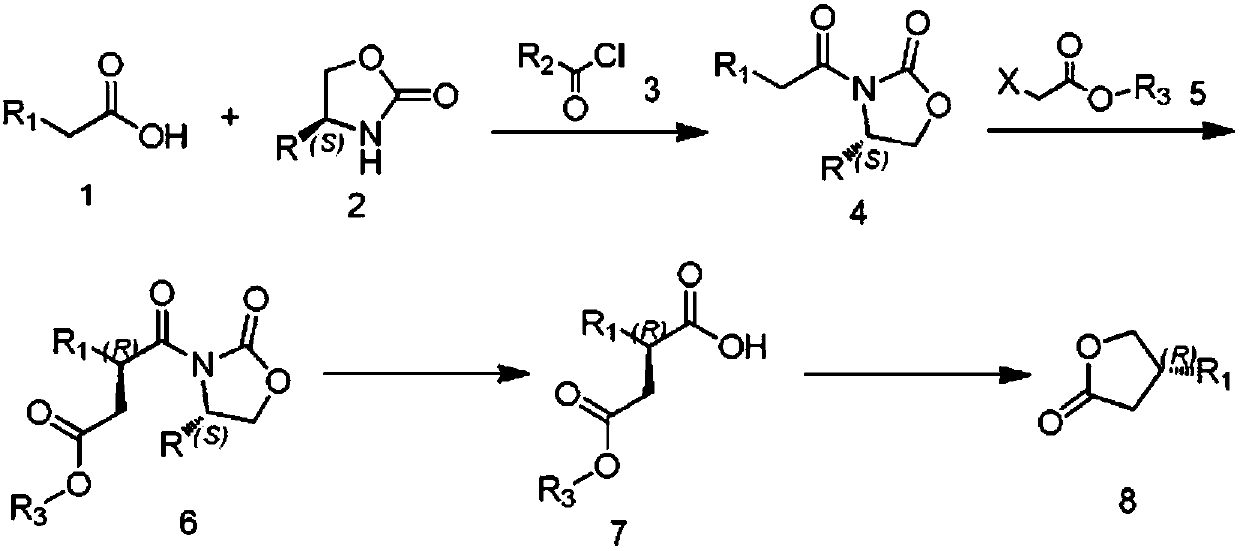
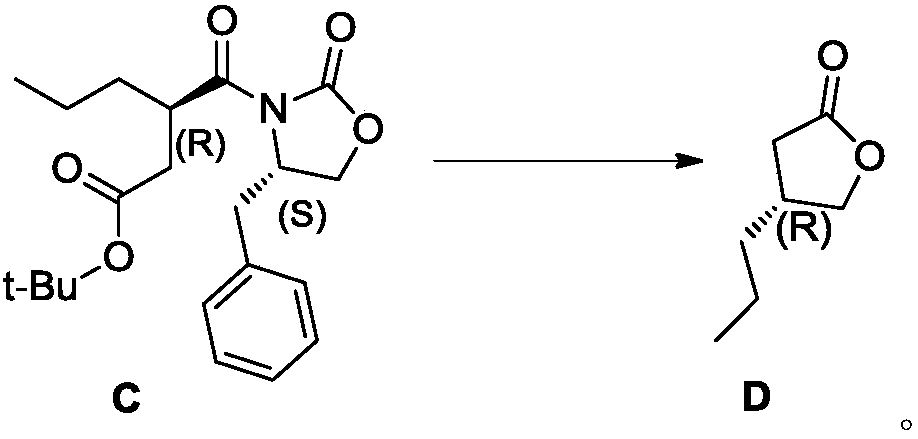
Preparation method of (R)-4-propyl-dihydrofuran-2-one
A technology of dihydrofuran and propyl, which is applied in the field of chemical drug synthesis, can solve the problems of limited industrial scale-up, high cost, and low yield, and achieve the effects of low cost, less waste gas, and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0074] The preparation of embodiment 1~5 formula B compound
[0075]
Embodiment 1
[0077] Into a 30L reaction kettle, add 428.31g of n-valeric acid and 6.0L of tetrahydrofuran, under stirring, add 951.23g of triethylamine, under the protection of nitrogen, cool down to -15°C, dropwise add 510.77g of pivaloyl chloride, dropwise, React at -20°C for 1.0h, add 187.21g of lithium chloride, keep warm for 10min, add dropwise a tetrahydrofuran solution (2.4L tetrahydrofuran) containing 600.0g of reactant A, and control the temperature at -20°C; , heated to 20°C, stirred for 12.0h, after the reaction, added 6.0L of 10% potassium carbonate aqueous solution, stirred for 10.0min, concentrated under reduced pressure to remove tetrahydrofuran, then added 6L of methyl tert-butyl ether for extraction; the water phase was reused 4.8L of methyl tert-butyl ether was extracted once, and finally the organic layers were combined, and the organic layer was washed once with 3.0L 1M hydrochloric acid and 3.0L aqueous sodium bicarbonate solution, and finally concentrated to remove the...
Embodiment 2
[0079] Into a 30L reactor, add 428.31g of n-valeric acid and 6.0L of 2-methyltetrahydrofuran, under stirring, add 743.54g of pyridine, under the protection of nitrogen, cool down to -15°C, dropwise add 510.77g of pivaloyl chloride, dropwise After completion, react at -5°C for 1.0h, add 187.21g of lithium chloride, keep warm for 10min, add dropwise 2-methyltetrahydrofuran solution (2.4L 2-methyltetrahydrofuran) containing 600.0g of reactant A, and control the temperature at -5°C After the dropwise addition was completed, after 2.0 hours of reaction, the temperature was raised to 20°C and stirred for 12.0 hours. After the reaction was completed, 6.0L of 10% potassium carbonate aqueous solution was added, and after stirring for 10.0 minutes, 2-methyltetrahydrofuran was removed by concentration under reduced pressure, and then added 6L methyl tert-butyl ether extraction; the aqueous phase was extracted once with 4.8L methyl tert-butyl ether, and finally the organic layer was combin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com