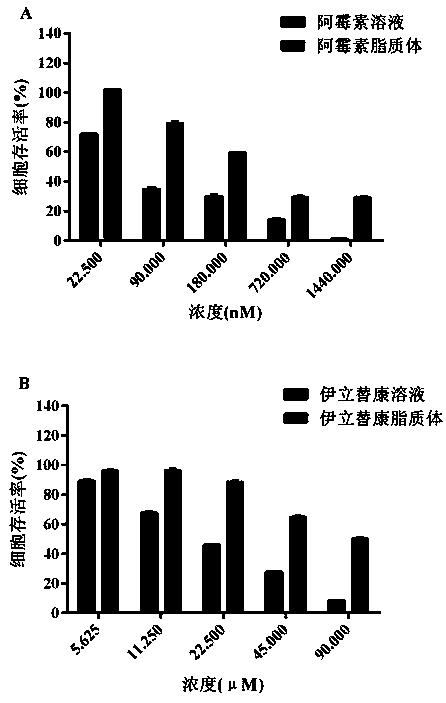
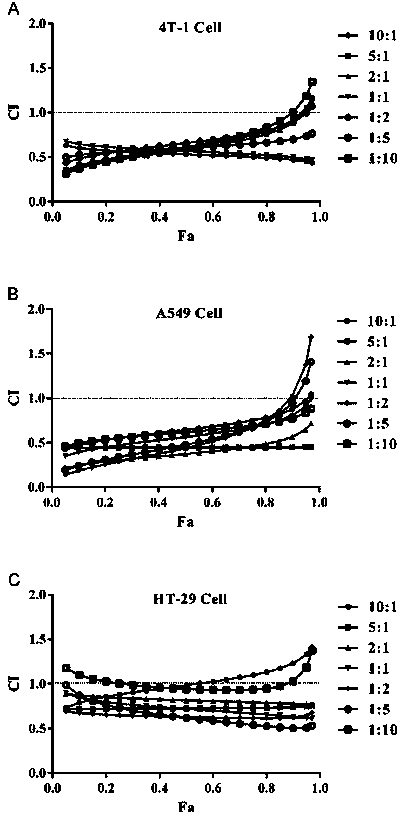
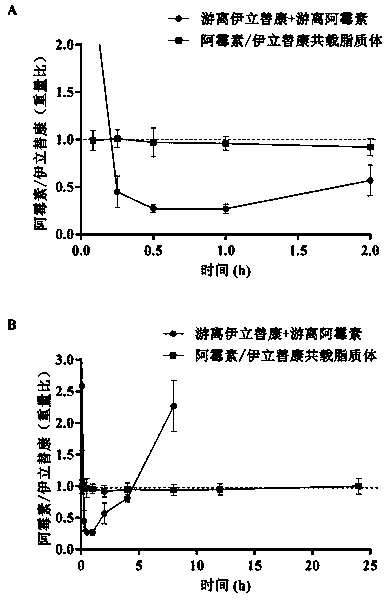
Irinotecan hydrochloride and doxorubicin hydrochloride co-loaded liposome and preparation method thereof
A technology of irinotecan hydrochloride and doxorubicin hydrochloride, which is applied in the field of irinotecan hydrochloride and doxorubicin hydrochloride co-loaded liposomes and its preparation, can solve the problems of toxicity and further research, and achieve delayed release, Improved drug delivery efficiency and high loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: film dispersion method
[0048] Combine DSPC, Cholesterol and MPEG 2000 -DSPE was mixed at a weight ratio of 3:1:0.05, dissolved in absolute ethanol, evaporated under reduced pressure to form a film, added 0.6M TEA-SOS solution, and hydrated at 65°C for 1 hour to obtain heterogeneous multilocular liposomes. Use high-pressure extrusion equipment to reduce the particle size of liposomes, then use Sephadex G-50 to remove anions from the outer phase of blank liposomes by column chromatography, and replace it with a solution containing 20mM HEPES and 150mM NaCl to form a transmembrane kinetic gradient. According to irinotecan: doxorubicin: phospholipid (weight ratio) is 0.4:0.4:1, first add irinotecan solution to blank liposomes, incubate at 65°C for 30min, then add doxorubicin solution and incubate for 30min, namely Co-loaded liposomes with irinotecan and doxorubicin.
Embodiment 2
[0049] Embodiment 2: ethanol injection method
[0050] Combine DSPC, Cholesterol and MPEG 2000 - DSPE is mixed in a weight ratio of 3:1:0.05, dissolved in absolute ethanol, stirred in a water bath at 65°C, evaporated to remove ethanol under reduced pressure, then added 0.6M TEA-SOS solution, hydrated at 65°C for 1 hour, and obtained inhomogeneous multilamellar liposomes. Use a high-pressure extrusion device to reduce the particle size of the liposomes, then use Sephadex G-50 to remove the anions of the outer phase of the blank liposome, and replace it with a solution containing 20mM HEPES and 150mM NaCl. According to irinotecan: doxorubicin: phospholipid (weight ratio) is 0.4:0.4:1, first add irinotecan solution to blank liposomes, incubate at 65°C for 30min, then add doxorubicin solution and incubate for 30min, namely Co-loaded liposomes with irinotecan and doxorubicin.
Embodiment 3
[0052] Combine DSPC, Cholesterol and MPEG 2000 - DSPE was mixed in a weight ratio of 3:1:0.1, dissolved in absolute ethanol, evaporated under reduced pressure to form a film, added 0.6M TEA-SOS solution, and hydrated at 65°C for 1 hour. The particle size was reduced using high-pressure extrusion equipment, and then Sephadex G-50 was used to remove anions from the outer phase of the liposome and replace it with a solution containing 20 mM HEPES and 150 mM NaCl. According to irinotecan: doxorubicin: phospholipid (weight ratio) is 0.4:0.4:1, load irinotecan solution first, then load doxorubicin solution, and incubate at 65°C to obtain irinotecan and doxorubicin co-loaded fat plastid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com