Cell-penetrating peptide and particles thereof coating hydrophobic molecules and preparation method and application of particles
A membrane-penetrating peptide and hydrophobic technology, applied in chemical instruments and methods, medical preparations of non-active ingredients, peptides, etc., can solve the problems that cannot meet the requirements of imaging or drug loading applications, limited number and types of molecules, and bonding process Complicated and other issues, to achieve the effect of reducing photobleaching, uniform size, and improving signal-to-noise ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of polypeptide sequences: SEQ ID NO: 4, SEQ ID NO: 5, SEQ ID NO: 6, SEQ ID NO: 7, SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 10 and SEQ ID NO: The peptides of the sequence shown in 11 are all composed of common amino acids and prepared by the classic solid-phase synthesis method. After these peptide sequences are purified by liquid chromatography, the purity can reach more than 95%.
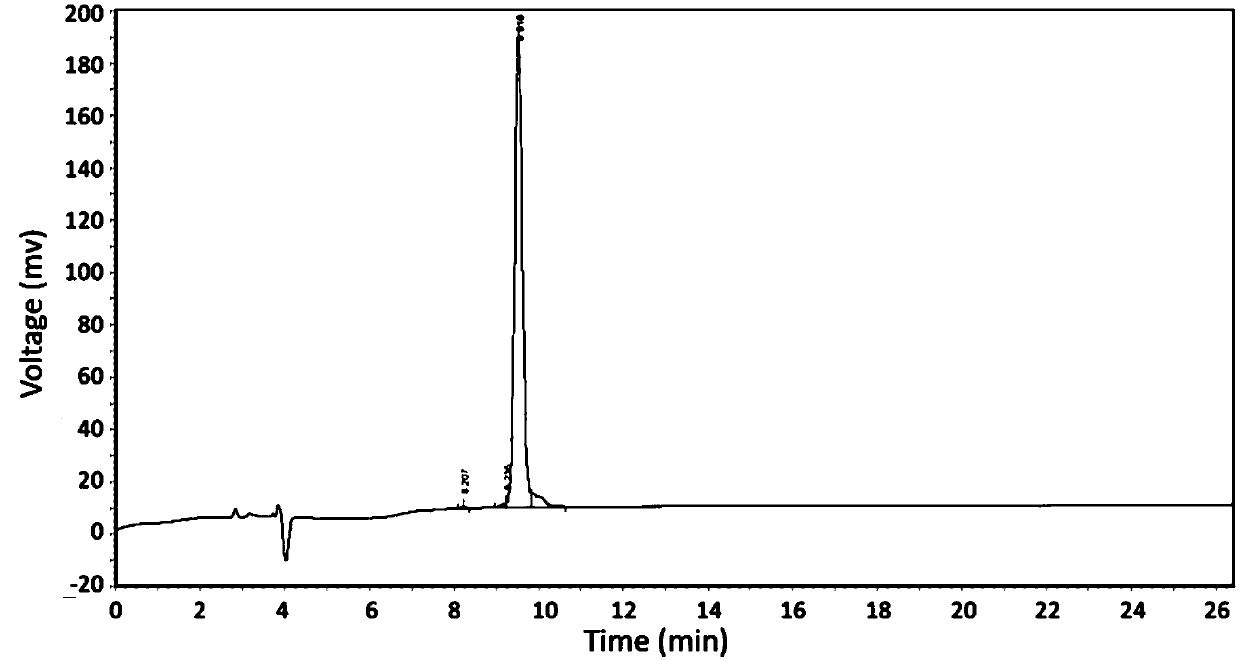
[0048] Wherein, the HPLC spectrogram of the purified SEQ ID NO: 4 is shown in figure 1 , the mass spectrum of the sequence: [M+3H] 3+ =831.79.
[0049] The mass spectra of the remaining peptides are as follows:
[0050] The mass spectrum of the sequence SEQ ID NO: 5 of the amphiphilic cell-penetrating peptide: [M+3H] 3+ =706.81
[0051] The mass spectrum of the sequence SEQ ID NO: 6 of the amphiphilic cell-penetrating peptide: [M+6H] 6+ =465.43
[0052] The mass spectrum of the sequence SEQ ID NO: 7 of the amphiphilic cell-penetrating peptide: [M+5H] 5+ =598.18
[0053] The m...
Embodiment 2
[0058] Preparation method of amphiphilic cell-penetrating peptide and nanoparticles coated with hydrophobic molecules
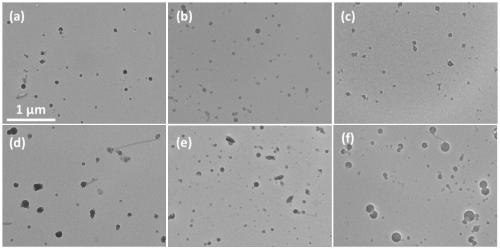
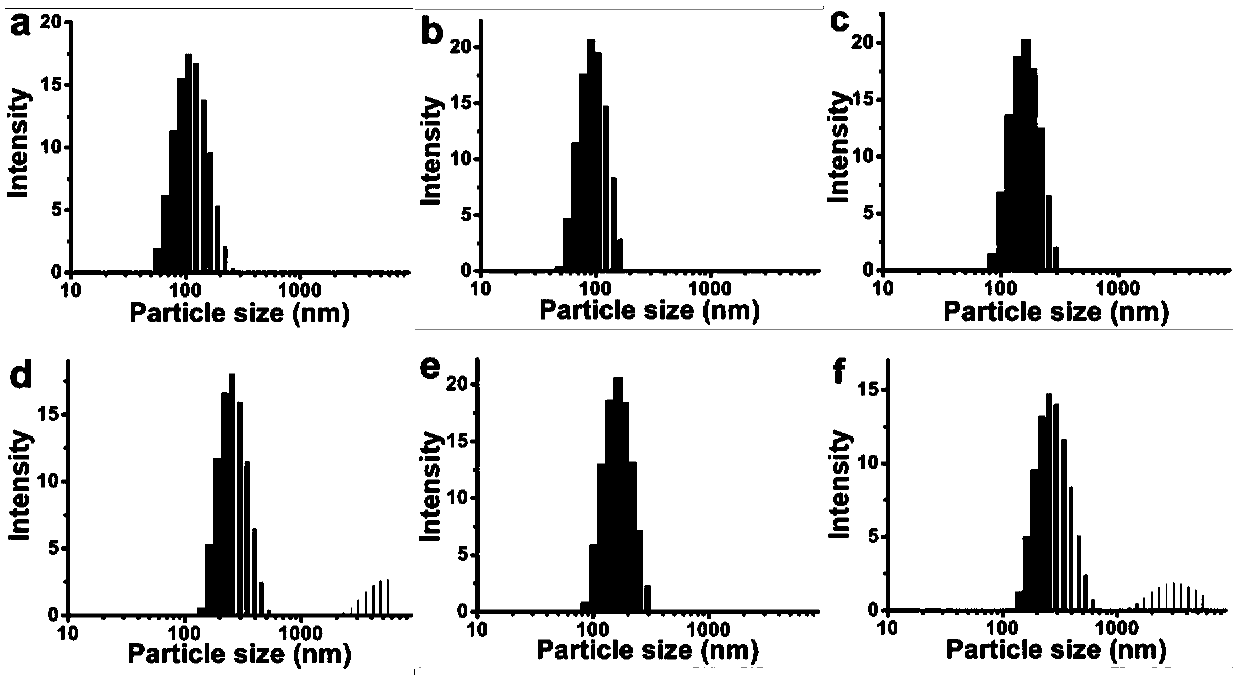
[0059] Take 25 μL of 1 mg / mL aqueous solution of sequence SEQ ID NO: 4, and slowly add it dropwise to 100 μL of 0.25 mg / mL hydrophobic fluorescent molecule Nile Red tetrahydrofuran solution. Good, uniformly sized nanoparticles. Its transmission electron microscope picture is shown in figure 2 (a), DLS diagram see image 3 (a).
[0060] Take 50 μL of 1 mg / mL aqueous solution of sequence SEQ ID NO: 5, and slowly add it dropwise to 100 μL of 0.25 mg / mL hydrophobic delayed luminescence molecule 4CzIPN (Ishimatsu Ryoichi, et al.Solvent effect on thermally activated delayed fluorescence by1,2,3,5 -tetrakis(carbazol-9-yl)-4,6-dicyanobenzene.Journal of Physical Chemistry A(2013)117(27):5607-5612) tetrahydrofuran solution, after it is ultrasonically uniform, add 900μL deionized water to obtain Nanoparticles with good dispersion and uniform size. Its transmission...
Embodiment 3
[0068] Cytotoxicity assay of amphiphilic cell-penetrating peptides and Nile red nanoparticles coated with MTT
[0069] The same number (1.5 × 10 4 / well) cells in a 96-well plate in complete medium, 37°C, 5% CO 2 The cells were cultured for 12 hours under the environment of the environment, and the cells adhered to the wall. Add 10 μL of the above-prepared Nile Red nanoparticles, mix well and then incubate at 37°C, 5% CO 2 Incubate for 24 hours under ambient conditions. After incubation, MTT (5 mg / mL) was added, followed by incubation for 4 h, the upper layer liquid was sucked off, and 100 μL DMSO solution was added. The absorbance of each well was detected at 570 nm by a microplate reader. Model of microplate reader: Tecan Infinite F50.
[0070] The cytotoxicity of amphiphilic cell-penetrating peptide and its coated Nile red nanoparticles was detected by MTT assay. Figure 4 (a) shows that after 24 hours of incubation with the amphiphilic cell-penetrating peptide, the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com