Preparation method of benzodithiocyclopentadiene derivative
A technology for benzodithiocyclopentadiene and derivatives, which is applied in the field of preparation of benzodithiocyclopentadiene derivatives, can solve the problems of narrow substrate range, harsh reaction conditions, lack of practical value and the like, and achieves Easy separation and purification, rich synthesis methods, and green and environmentally friendly reaction systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
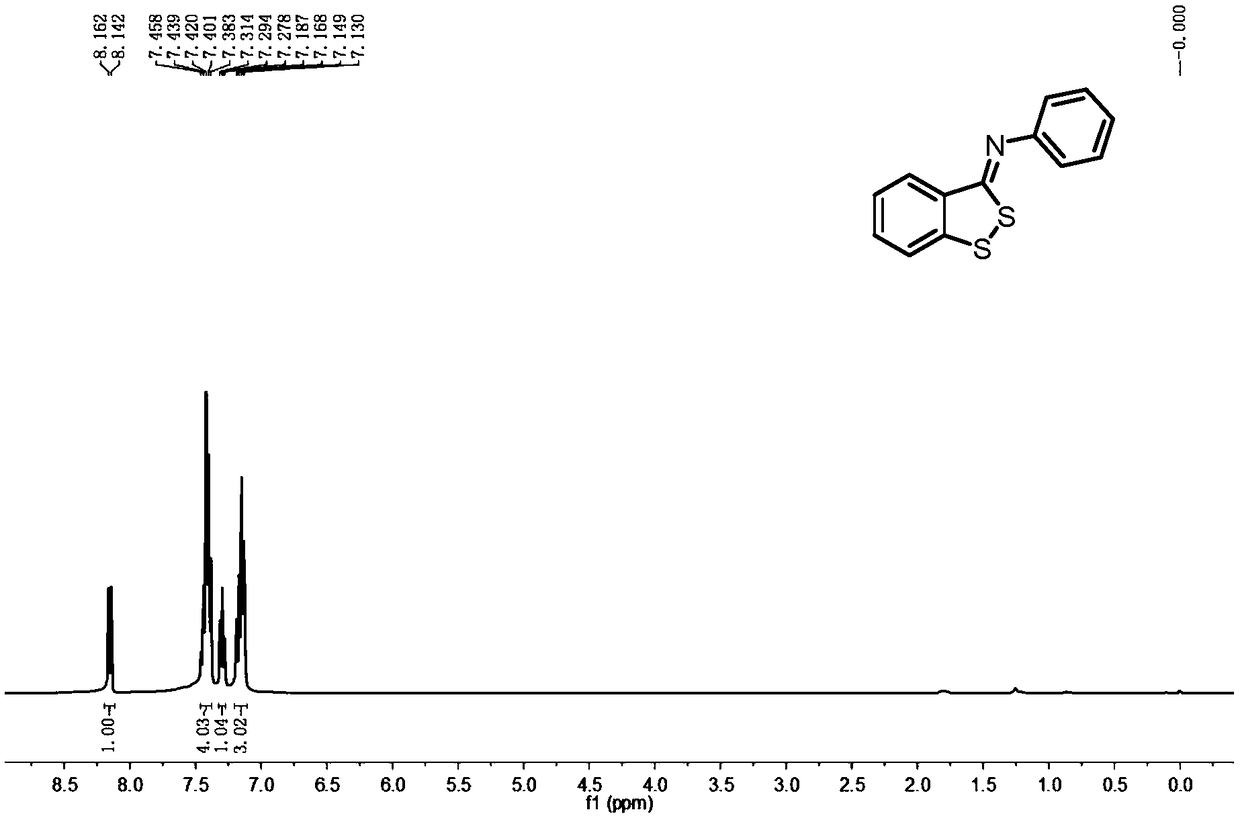
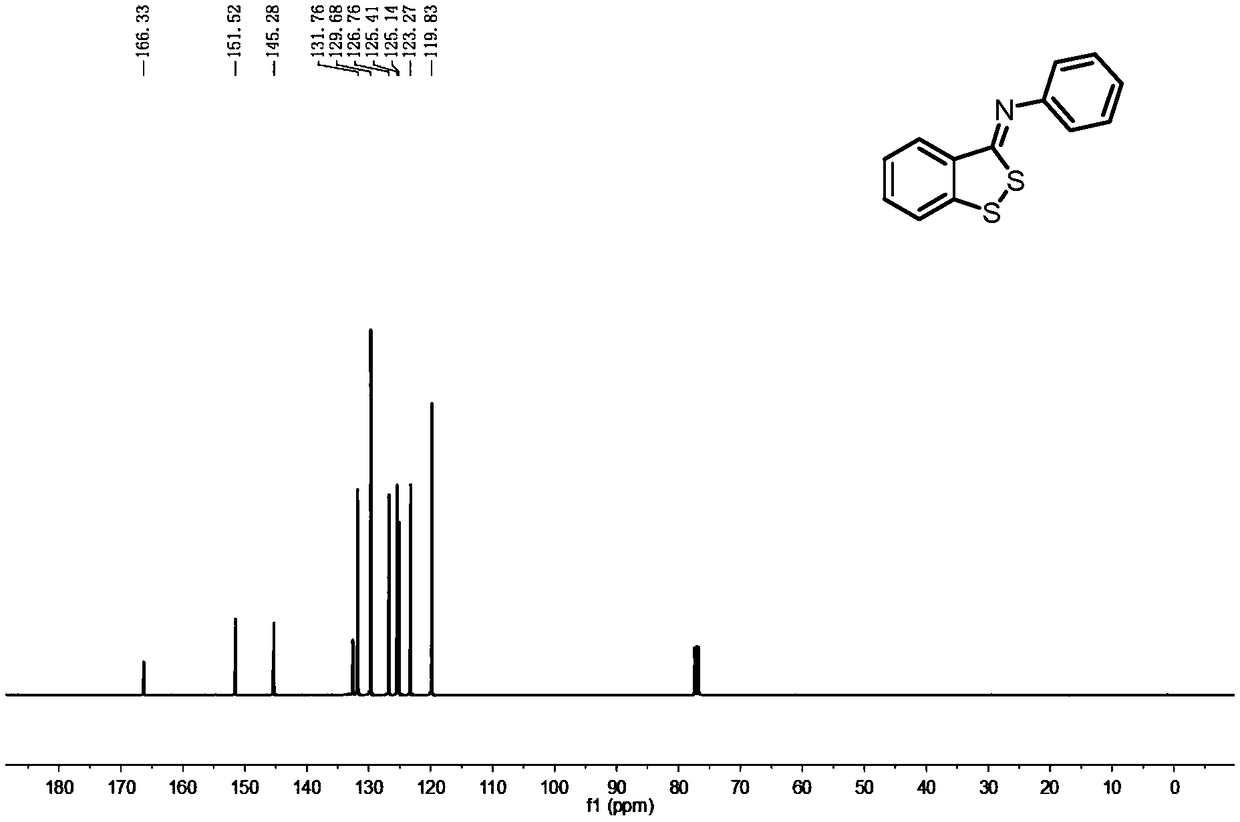
[0029] Example 1: Preparation of benzodithiocyclopentadiene derivative 2a, the experimental results are shown in Table 1.
[0030]
[0031] To a 50 mL Shrek bottle with a magnetic stirring device was added toluene (10 mL), N-phenyl-2-bromothiobenzamide 1a (0.291 g, 1.0 mmol) and S 8 (0.036g, 1.2mmol), add cuprous iodide (0.019g, 0.1mmol), 1,10-phenanthroline (0.036g, 0.2mmol), cesium carbonate (0.326g, 1.0mmol) after stirring, will It was placed in a 100°C oil bath and continued to stir. TLC detects that the substrate disappears, and the reaction ends. The reaction solution was poured into saturated aqueous sodium chloride solution (10mL), extracted with dichloromethane (3×10mL), and the organic phases were combined, then backwashed with water (3×10mL), dried over anhydrous calcium chloride, Steps such as suction filter, vacuum distillation obtain viscous solid, finally through silica gel column chromatography (eluent is V 石油醚 :V 乙酸乙酯 =20:1) A yellow solid was obtained,...
Embodiment 2
[0035] Replace 1a in Example 1 with 1b, wherein the amount of each material is: 1b (0.306g, 1.0mmol) and S 8 (0.036g, 1.5mmol), add cuprous iodide (0.19g, 1mmol), 1,10-phenanthroline (0.108g, 0.6mmol), cesium carbonate (0.65g, 2.0mmol); The non-aqueous solvent Select DMF (10 mL).
[0036] Other conditions are the same as Example 1, and the experimental results are shown in Table 1.
[0037]
[0038] Spectrum analysis data 2b:
[0039] Yellow solid, m.p.164-165℃; 1 H-NMR (400MHz, CDCl 3 )δ8.15(d,J=8.8Hz,1H),7.47-7.42(m,2H),7.32-7.28(m,1H),7.21(d,J=8.0Hz,2H),7.05(d,J =7.2Hz, 2H), 2.35(s, 2H); 13 C-NMR (CDCl 3,100MHz)δ164.8,147.9,144.2,133.8,131.7,130.7,129.2,125.7,124.4,122.3,118.8,20.0; HRMS(APCI)m / z calculated for C 14 h 11 NS 2 [M+H] + :258.0406found:258.0411.
Embodiment 3
[0041] Replace 1a in Example 1 with 1c, wherein the amount of each material is: 1c (0.312g, 1.0mmol) and S 8 (0.072g, 3mmol), add cuprous iodide (0.19g, 1mmol), 1,10-phenanthroline (0.18g, 1mmol), cesium carbonate (0.65g, 2.0mmol); Described non-aqueous solvent selects DMF (10 mL).
[0042] Other conditions are the same as Example 1, and the experimental results are shown in Table 1.
[0043]
[0044] Spectral analysis data 2c:
[0045] Yellow solid, m.p.173-174℃; 1 H-NMR (400MHz, CDCl 3 )δ8.18-8.15(m,1H),7.52-7.47(m,2H),7.37-7.33(m,1H),7.16-7.12(m,2H),6.99-6.95(m,2H),3.84( s,3H); 13 C-NMR (CDCl 3 ,100MHz)δ165.6,157.1,145.1,144.7,132.9,131.7,126.8,125.5,123.4,121.4,114.8,55.5; HRMS(APCI)m / z calculated for C 14 h 11 NOS 2 [M+H] + :274.0355found:274.0381.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com