Chiral polyamide film and preparation method and application thereof
A polyamide membrane and chiral technology, applied in the field of chiral separation, can solve the problems of high energy consumption, low efficiency, and large pollution of the resolution method, and achieve wide tolerance to pH, wide range of resolution objects, and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
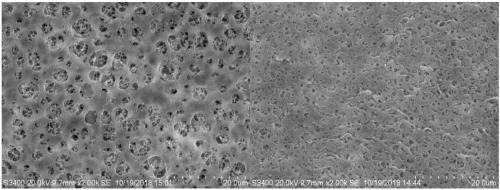
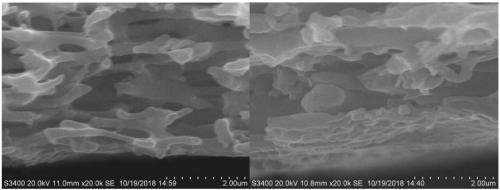
Image
Examples
Embodiment 1
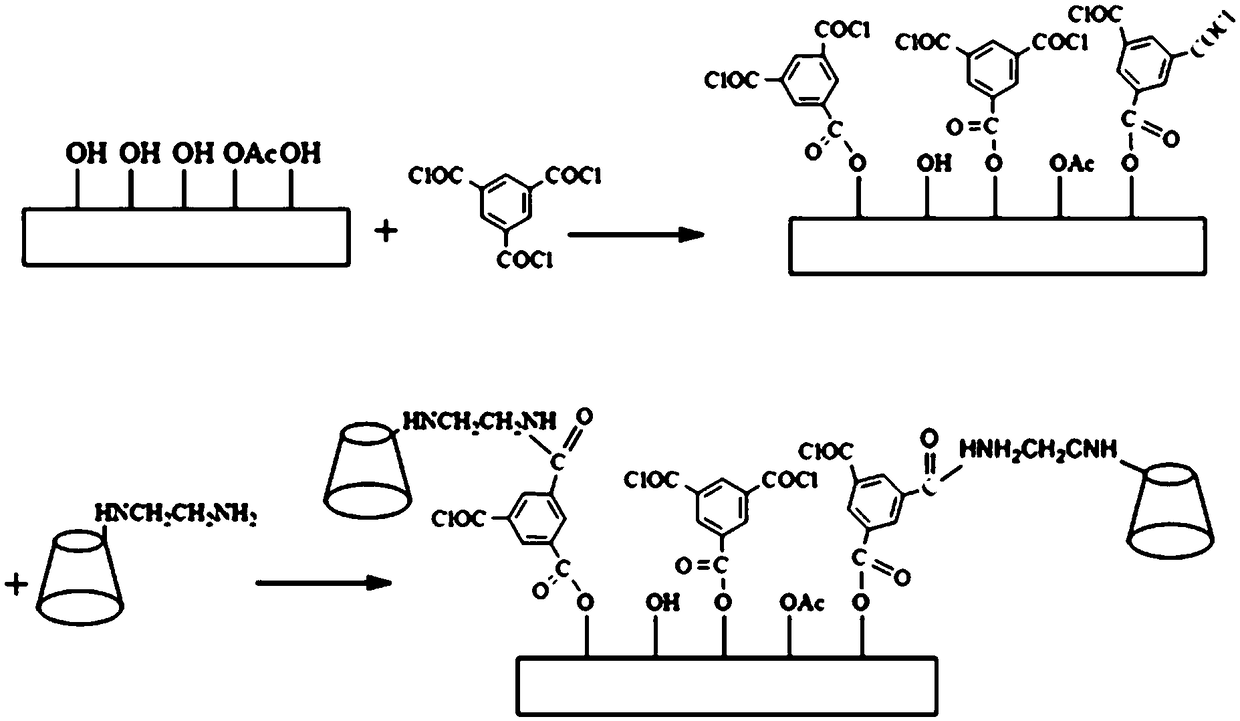
[0036] First prepare the ethylenediamine-β-cyclodextrin chiral polyamide membrane by the following steps, and then detect the concentration ratio and The concentration ratio of the two configurations in the stock solution was used to evaluate its resolution performance.
[0037] Synthesis of ethylenediamine-β-cyclodextrin:
[0038]The recrystallized β-cyclodextrin (100 g, 88 mmol) was weighed into a 1000 mL beaker containing 833 mL of purified water. NaOH (10.95g, 0.27mol) was dissolved in 33mL of water, and was added dropwise to the above suspension within 10min under magnetic stirring. After the addition was complete, the suspension became a uniform and slightly yellow solution. Weigh p-toluenesulfonyl chloride (p-TsCl, 16.82g, 0.09mol) and dissolve it in 50mL of acetonitrile, and add the solution dropwise to the above-mentioned light yellow reaction solution containing β-cyclodextrin. After 75min, all the drops are completely added, and there is A large amount of white pr...
Embodiment 2
[0053] A preparation method of ethylenediamine-beta-cyclodextrin, comprising the following steps:
[0054] (1) Weigh the recrystallized β-cyclodextrin (90g, 79mmol) and add it into a 1000mL beaker containing 800mL of pure water, dissolve NaOH (10g, 0.25mol) in 30mL of water, and under magnetic stirring conditions, Add it dropwise to the above suspension within 8 minutes;
[0055] (2) After the β-cyclodextrin suspension turns into a clear light yellow solution, weigh p-toluenesulfonyl chloride (p-TsCl, 16g, 0.086mol) and dissolve it in 45mL of acetonitrile, and add the solution dropwise to the above In the light yellow reaction solution of β-cyclodextrin, after 70 minutes, all the drops were completed, and a large amount of white precipitate was formed;
[0056] (3) The above reaction system was vigorously stirred at room temperature for 2h, and then 2mol·L -1 The hydrochloric acid was adjusted to nearly neutral, and placed in a refrigerator at 4°C overnight; the next day, su...
Embodiment 3
[0068] A preparation method of ethylenediamine-beta-cyclodextrin, comprising the following steps:
[0069] (1) Weigh recrystallized β-cyclodextrin (110g, 96.8mmol) and add it into a 1000mL beaker containing 850mL of purified water, dissolve NaOH (12g, 0.3mol) in 40mL of water, and under magnetic stirring conditions, Add it dropwise to the above suspension within 11 minutes;
[0070] (2) After the β-cyclodextrin suspension turns into a clear light yellow solution, weigh p-toluenesulfonyl chloride (p-TsCl, 18g, 0.096mol) and dissolve it in 50mL of acetonitrile, and add the solution dropwise to the above In the light yellow reaction solution of β-cyclodextrin, after 80 minutes, all the drops were completely added, and a large amount of white precipitate was formed;
[0071] (3) The above reaction system was vigorously stirred for 3 hours at room temperature, and then 2mol L -1 The hydrochloric acid was adjusted to nearly neutral, and placed in a refrigerator at 4°C overnight; t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com