qx type ibv hemagglutination inhibition test antigen and its preparation method and application
A technology of hemagglutination inhibition test and antigen, which is applied in the biological field, can solve the problems of inability to carry out QX type IBV, decrease in accuracy rate, and distant evolutionary relationship, and achieve improved reliability and operability, high specificity, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation and Concentration of Example 1 IBV SZ Strain Virus Liquid
[0050] (1) Propagation of the IBV SZ strain: the allantois of the IBV SZ strain preserved at -80°C was passed through the allantoic cavity, according to 10 5 EID 50 Inoculate 9-11 day-old SPF chicken embryos, 0.2mL / embryo. Incubate in a constant temperature incubator at 37°C, observe once every 6 hours, discard the dead embryos within 24 hours, take out the chicken embryos after 36 hours of incubation, cool at 4°C for 10 hours, and aseptically collect the allantoic fluid of the chicken embryos. Viral allantoic fluid was used or stored in a -80°C refrigerator for later use.
[0051] (2) Concentration of IBV SZ strain: the IBV virus allantoic fluid is centrifuged at 8000rpm for 45min, and the supernatant is obtained; centrifuged at 40000rpm for 2 hours with a high-speed centrifuge, the supernatant is discarded, and the IBV virus fluid (supernatant) is used for precipitation after the first centrifug...
Embodiment 2
[0052] Example 2 Exploration of the action time of type I phosphatase C (PLC1)
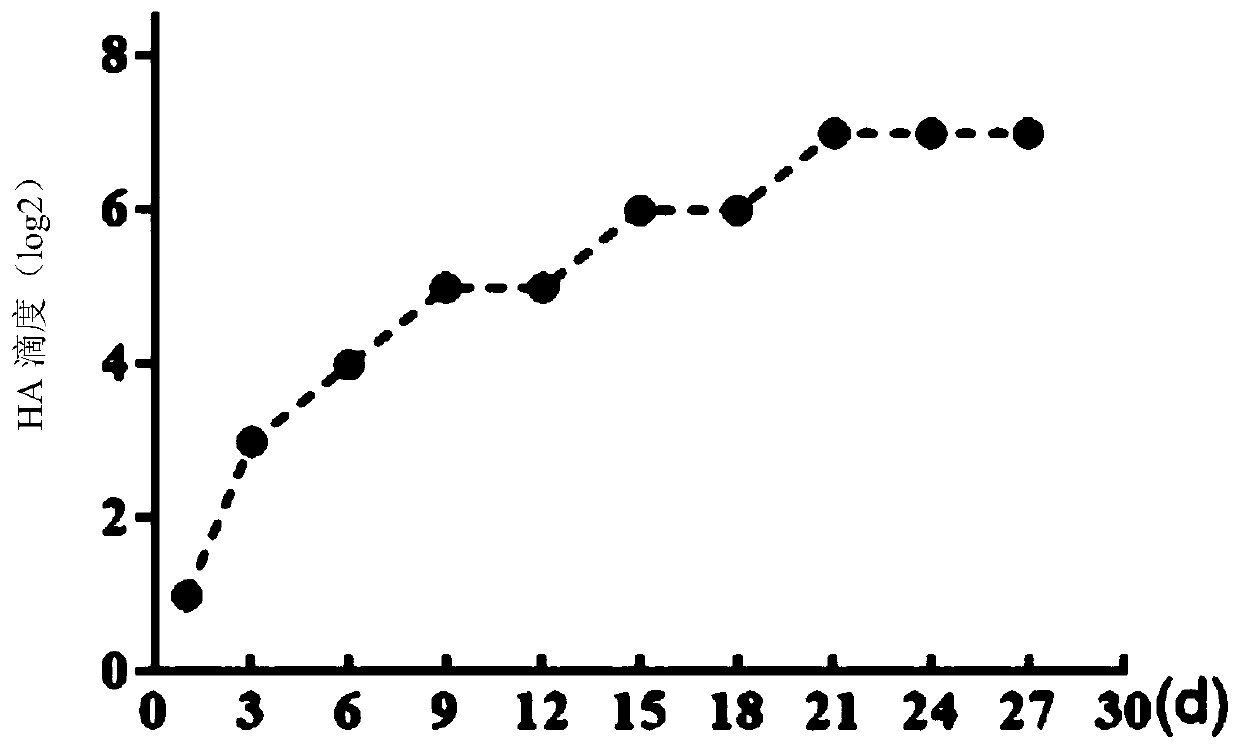
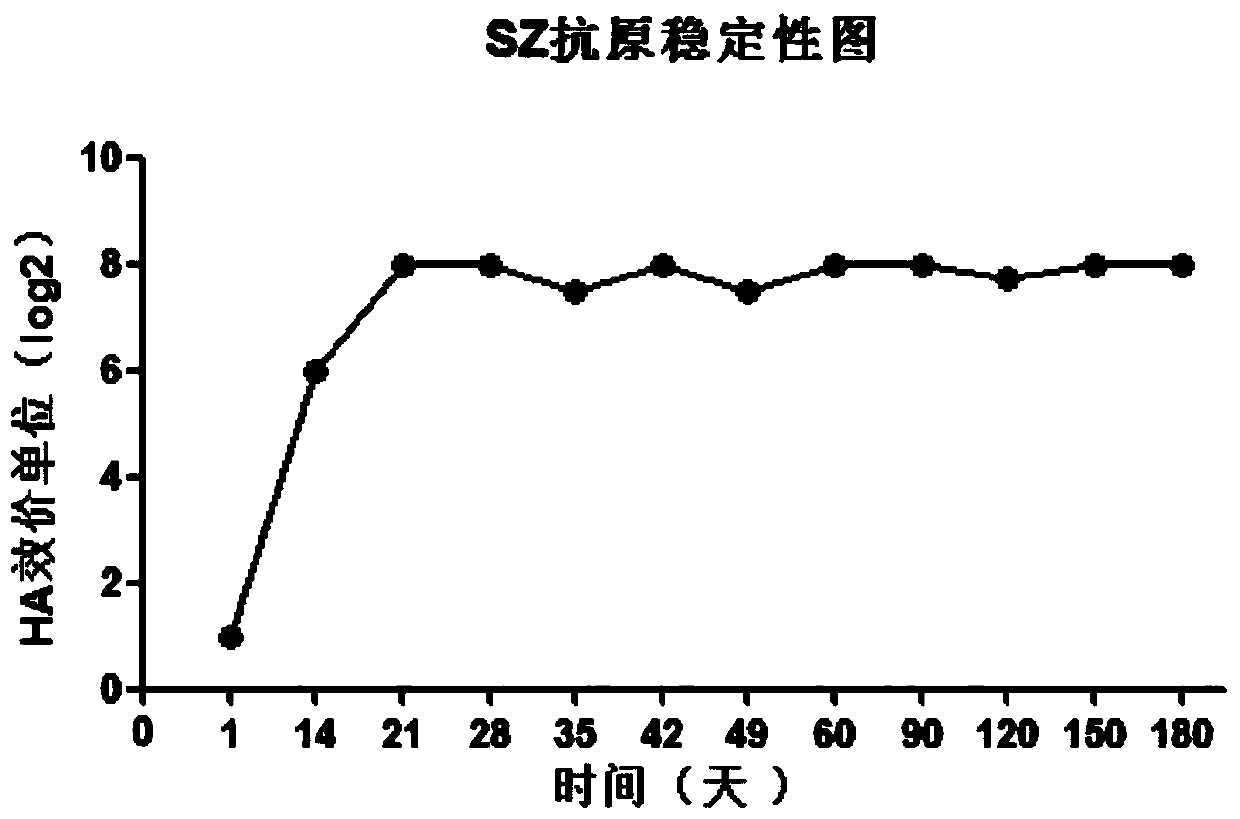
[0053] Preparation of hemagglutination inhibitory antigen of IBV SZ strain: Add 200-fold concentrated IBV virus liquid to type I phosphatase C (PLC1) to make the final concentration of PLC1 2.5U / mL, mix well, and then place the virus liquid in 37°C constant temperature oscillation 200r / min for 2 hours, take it out at 4°C and place it for several days for treatment, every 3 days, measure the hemagglutination titer of the antigen, the test results show that the concentrated antigen HA titer of the IBV QX type SZ strain after PLC1 treatment Increased with the different storage time at 4°C ( figure 1 ), after 21 to 35 days, the titer of HA was the highest and remained stable, and the IBV-HI antigen was obtained. The results of hemagglutination titer determination are shown in Table 1 (the titer reaches 1:256 from 21 days to 35 days):
[0054] Table 1 Hemagglutination titer of IBV SZ strain hemagglut...
Embodiment 3
[0056] Example 3 Optimum reaction temperature and time optimization of IBV SZ strain HI antigen HA test
[0057] Utilize the antigen that embodiment 1 and 2 prepare, the condition determined with reference to embodiment is to the optimal reaction temperature of IBV SZ strain HI antigen HA test and the optimal operation method of time as follows:
[0058] IBV-HI antigen HA test operation:
[0059] ① Carry out on a 96-well microplate, from the 1st well to the 12th well, add 25 μL of normal saline to each well.
[0060] ② Add 25 μL of the HI antigen prepared in Example 1 to the first well on the left side, mix well, then pipette 25 μL into the second well, then serially dilute to the 12th well, and discard 25 μL.
[0061] ③ Add 25 μL of 0.5% fresh chicken erythrocyte suspension to each well successively from right to left, and shake on the shaker for 30 seconds.
[0062] Then place the hemagglutination plate under different conditions respectively, at 2-8°C for 30 minutes; at 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com