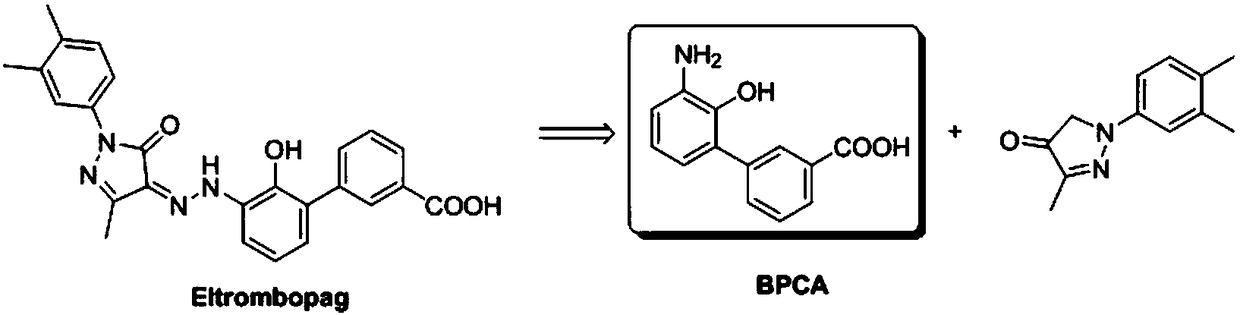
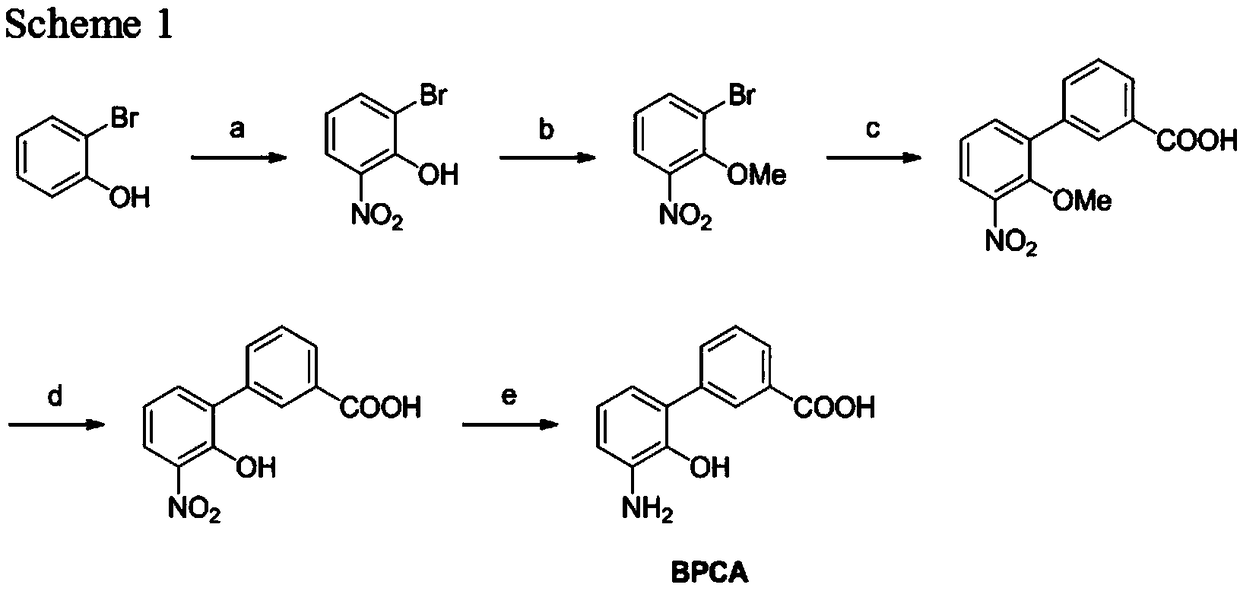
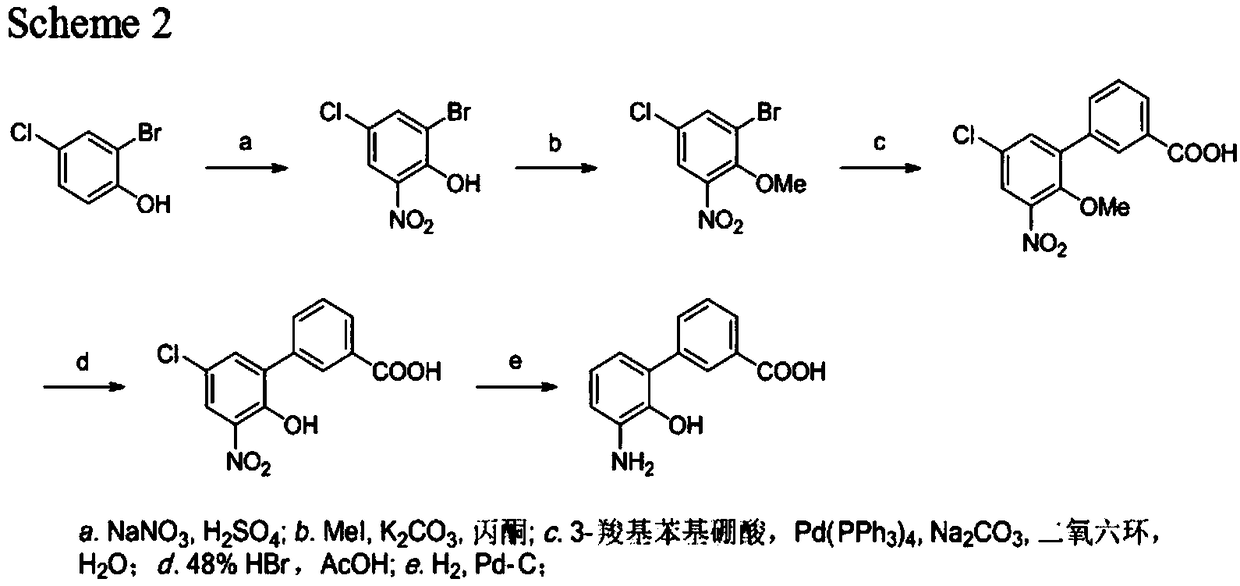
Synthetic method of eltrombopag intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve the problems of high environmental pressure, high cost, and many reaction steps, and achieve the effects of less waste disposal, fewer reaction steps, and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] One of the preparation method of embodiment 1 BPCA:
[0056] Add 10g N-(2-hydroxyphenyl) benzyl carbamate, 9g 3-bromobenzoic acid, 0.73gRhCl(cod) in a 250ml reaction flask 2 , 1.28g triphenoxyphosphine, 40g cesium carbonate and 100ml toluene, reflux reaction under the protection of nitrogen, TLC monitors that the reaction is complete. Add 100ml of water, extract with ethyl acetate, dry and concentrate to obtain the product (13g, yield: 87%, HPLC purity: 97%).
[0057] Add the above product into a hydrogenation kettle, add 2g of 10% palladium carbon (about 63% of water content), and 150ml of methanol, and stir at room temperature by feeding hydrogen gas at 0.1-0.3MPa. After the reaction was complete, filter, concentrate, add 100ml of water to dissolve, adjust the pH to about 5.5 with 1M hydrochloric acid, precipitate a solid, filter with suction, and dry to obtain a reddish-brown solid (9g, yield: 95%, HPLC purity: 99%).
[0058] 1H NMR (600MHz, DMSO-d6): δ6.50(dd, J=1...
Embodiment 2
[0059] One of the preparation methods of embodiment 2 BPCA:
[0060] Add 10 grams of N-(2-hydroxyphenyl) tert-butyl carbamate, 13g 3-iodobenzoic acid, 0.85g RhCl(cod) in a 250ml reaction flask 2 , 1.5g triphenoxyphosphine, 46g cesium carbonate and 100ml toluene, reflux reaction under the protection of nitrogen, TLC monitors that the reaction is complete. Add 100ml of water, extract with ethyl acetate, dry and concentrate to obtain the product (13g, yield: 83%, HPLC purity: 97%).
[0061] The above product was added in 100ml of water, 120ml of 1M hydrochloric acid and 4.4g of thiophenol were added and stirred at room temperature for 5-8 hours. After the reaction was completed, it was filtered, and the solid was washed with 30ml of ethanol to obtain a light yellow solid (9.5g, yield: 90%, HPLC Purity: 99%)
[0062] 1H NMR (600MHz, DMSO-d6,): δ6.52(dd, J=1.8, 7.2Hz, 1H), 6.67~6.73(m, 2H), 7.52(t, J=7.8Hz, 1H), 7.72~ 7.74(m,1H),7.87(dt,J=1.2,7.8Hz,1H),8.09(t,J=1.2Hz,1H),MS m / z:...
Embodiment 3
[0063] One of the preparation methods of embodiment 3 BPCA:
[0064] In the 250ml reaction flask, add 10g 2-nitrophenol, 20g 3-iodobenzoic acid, 1.3g RhCl (cod) 2 , 1.3g tris(dimethylamino)phosphine, 40g potassium carbonate and 100ml xylene were reacted at 120°C under the protection of nitrogen, and the reaction was monitored by TLC to be complete. Add 100ml of water, extract with ethyl acetate, dry and concentrate to obtain the product (12g, yield: 65%, HPLC purity: 98%).
[0065] Add the above product into a hydrogenation kettle, add 2g of 10% palladium carbon (about 63% of water content), and 150ml of ethanol, and feed hydrogen gas at 0.5-1.0MPa / 50°C for reaction. After the reaction was completed, filter, concentrate, add 100ml of water to dissolve, adjust the pH to about 5.5 with 1M hydrochloric acid, precipitate a solid, filter with suction, and dry to obtain a light yellow solid (9g, yield: 95%, HPLC purity: 99%).
[0066] 1H NMR (600MHz, DMSO-d6,): δ6.51(dd, J=1.8, 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com