Artesunate heparin derivative as well as pharmaceutical composition and application thereof
A technology of succinate heparin and artesunate, which is applied in drug combinations, anti-infective drugs, pharmaceutical formulations, etc., can solve the problems of short half-life, poor water solubility of artemisinin and dihydroartemisinin, and low bioavailability , to achieve the effect of low toxicity, easy entry into the cell, and prolonging the half-life of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
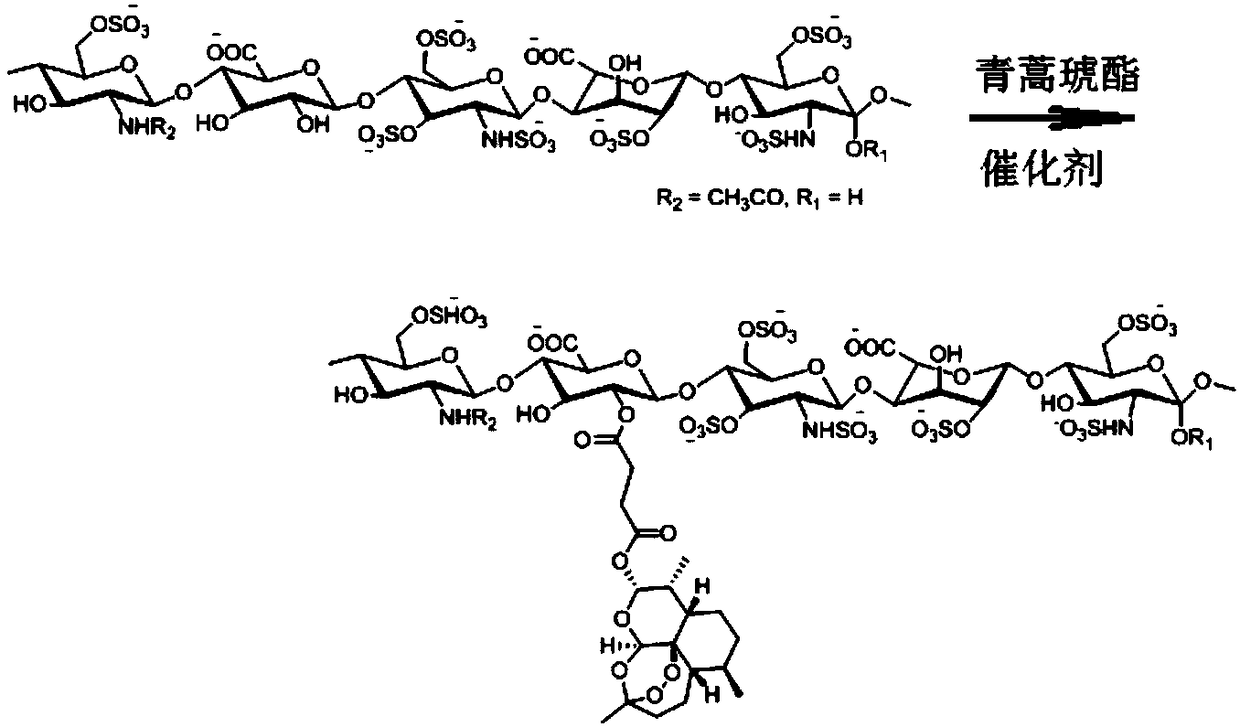
[0052] The preparation method of the artesunate heparin derivative of the present invention: the carboxyl group of the artesunate is activated and then reacted with heparin to connect them through an ester bond to obtain an amphiphilic artesunate heparin derivative. The amount of artesunate bound to the heparin molecule was measured using UV spectroscopy. The mass content of artesunate in the artesunate heparin derivative is 5%-50%.
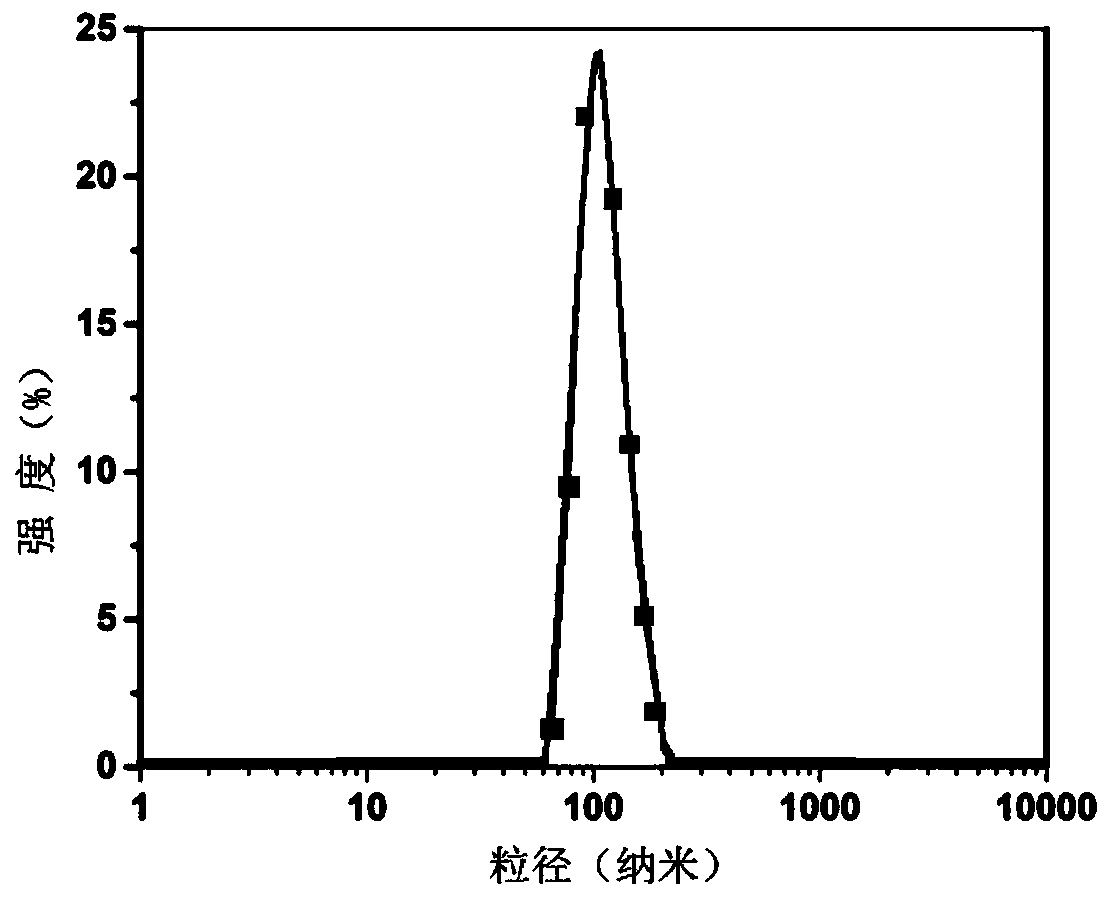
[0053] The preparation method of artesunate heparin derivatives and pharmaceutical composition nanoparticles: from the mixture of artesunate heparin derivatives and auxiliary agents or synergists of the present invention, through film dispersion method, reverse phase evaporation method, ultrasonic dispersion method, spray drying method or high pressure homogenization method and other methods.
[0054] The artesunate heparin derivative of the present invention has amphiphilicity, and the mixture of the artesunate heparin derivative of the present...
Embodiment 1
[0057] Synthesis of artesunate heparin derivatives (synthetic route see figure 1 )
[0058] Add 150 mg of heparin sodium (HEP) into 10 ml of dimethylacetamide (DMAc), heat to 50°C, stir for 3 hours, and dissolve for later use. 0.768 g (2 mmol) of artesunate (ART) was dissolved in 10 ml of dimethylacetamide, 0.324 g (2 mmol) of N,N'-carbonyldiimidazole (CDI) was added, and activated at room temperature for 2 hours. Then, the activated artesunate solution was added dropwise to the heparin solution, 0.122 g (1 mmol) of 4-dimethylaminopyridine (DMAP) was added, and the reaction was stirred at 30° C. for 48 hours. Add KH to the reaction liquid 2 PO 4 The solution was neutralized and dialyzed with distilled water (MWCO: 3500) for 24 hours to remove small molecule impurities. The white solid product of artesunate heparin derivative was obtained by freeze-drying, which was characterized by infrared spectrum and nuclear magnetic resonance spectrum. The mass percent content of arte...
Embodiment 2
[0060] Synthesis of Artesunate Heparin Derivatives
[0061] The molar ratio of reagents in this experiment was set as ART:CDI:DMAP=2:2:1. Add 30, 90, 180, and 270 mg of heparin sodium (HEP) into dimethylacetamide (DMAc), heat to 50°C, stir for 3 hours, and dissolve for later use. An appropriate amount of artesunate (ART) was dissolved in dimethylacetamide, and the corresponding molar amount of CDI was added, and activated at room temperature for 2 hours. Then, the activated artesunate solution was added dropwise to the heparin solution, an appropriate amount of DMAP was added, and the reaction was stirred at 30° C. for 48 hours. Add KH to the reaction liquid 2 PO 4 The solution was neutralized and dialyzed with distilled water (MWCO: 3500) for 24 hours to remove small molecule impurities. Freeze-drying obtained four white solid products of artesunate heparin derivatives, wherein the mass percentages of artesunate were 5.1%, 18.5%, 35.3%, and 49.6%, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com