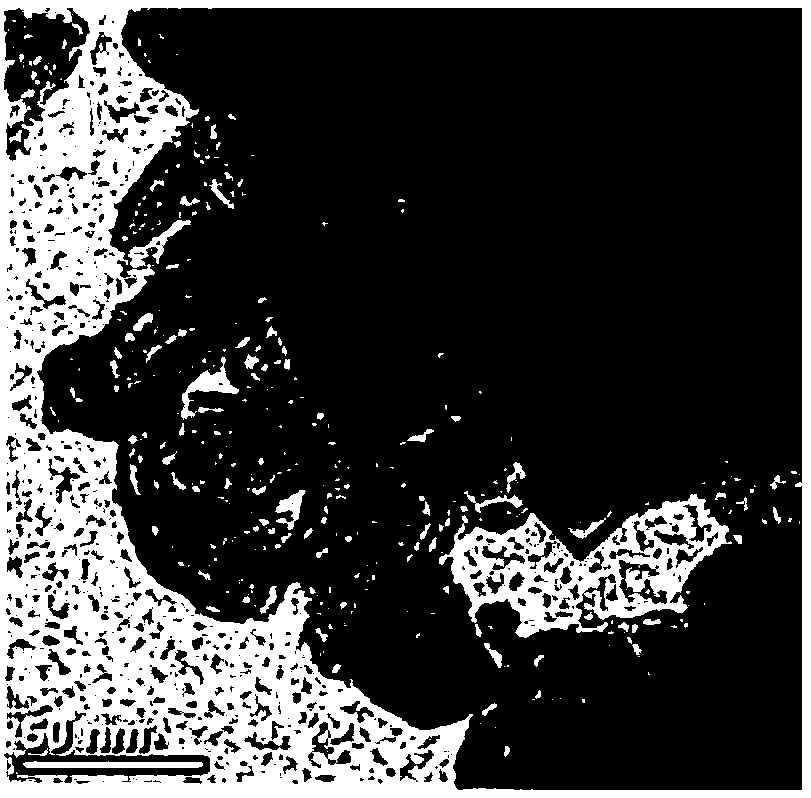
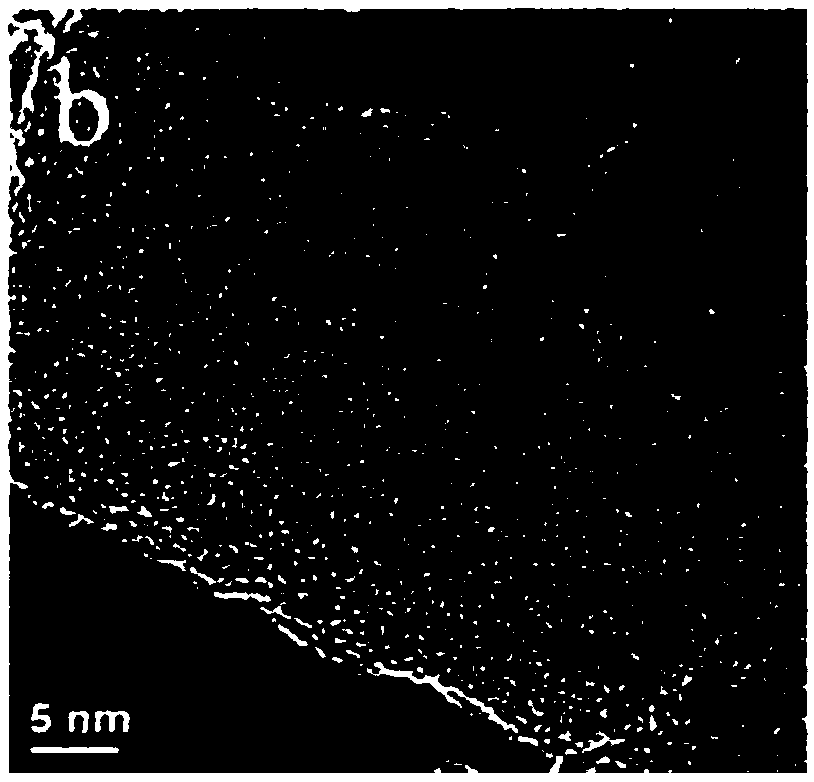
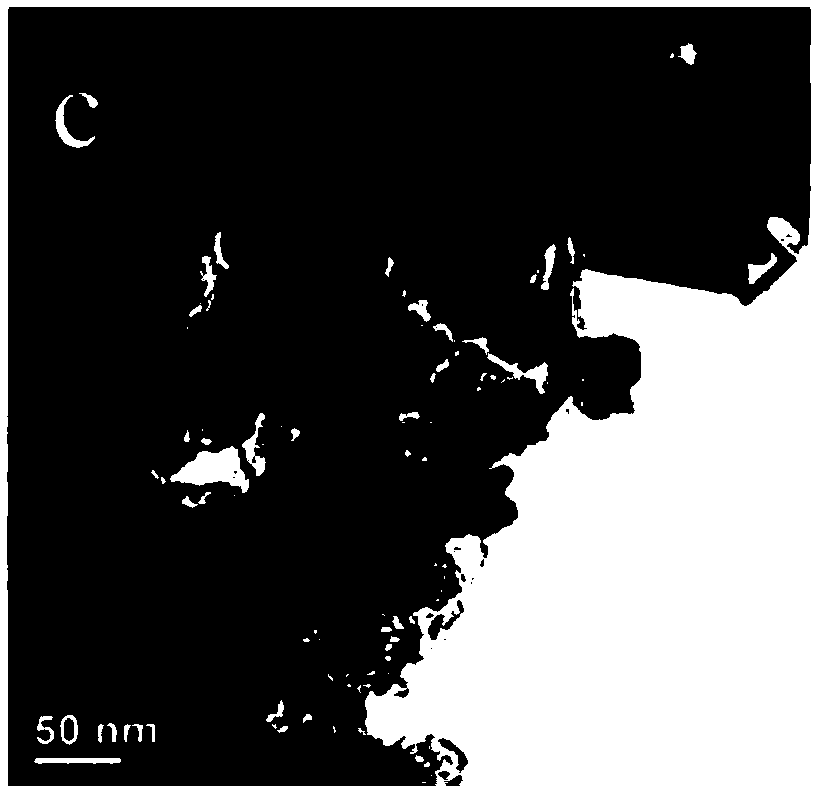
Preparation method and application of cubic FeOOH or Fe4(Fe(CN)6)3 loaded on nanocarbon ribbon
A cubic and nano-carbon technology, applied in the field of electrochemistry, can solve the problem of easy loss of resistance of catalytic sites, and achieve the effects of simple preparation method, promotion of catalytic performance, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]This embodiment discloses a preparation method for growing square-shaped FeOOH on a carbon tape, which is prepared by the following steps:
[0037] S1, the cation exchange resin was mixed with different proportions of transition metal salt FeCl 3 ·6H 2 O mixed to obtain mixture 1 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:1), mixture 2 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:2) and mixture 3 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:4); mixture 4 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:6); mixture 5 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:8); mixture 6 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:10);
[0038] S2. Add a certain amount of deionized water to the mixture in step S1, and stir at room temperature;
[0039] S3, filtering the mixture in step S2, drying, and pulverizing;
[0040] S4, the product gained in step S3 is put into qua...
Embodiment 2
[0043] This embodiment discloses a kind of carbon belt growing square-shaped Fe 4 (Fe(CN) 6 ) 3 The preparation method is prepared by the following steps:
[0044] S1, the cation exchange resin was mixed with different proportions of transition metal salt FeCl 3 ·6H 2 O mixed to obtain mixture 1 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:1), mixture 2 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:2) and mixture 3 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:4); mixture 4 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:6); mixture 5 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:8); mixture 6 (cation exchange resin: FeCl 3 ·6H 2 The mass ratio of O is 1:10);
[0045] S2. Add a certain amount of deionized water to the mixture in step S1, and stir at room temperature;
[0046] S3, filtering the mixture in step S2, drying, and pulverizing;
[0047] S4, the product gained in step S3 is...
Embodiment 3
[0054] This embodiment discloses a carbon ribbon growing square FeOOH or Fe 4 (Fe(CN) 6 ) 3 The preparation method is obtained by the following preparation steps:
[0055] S1, the cation exchange resin was mixed with different proportions of transition metal salt FeSO 4 Mix to obtain a mixture (cation exchange resin: FeSO 4 The mass ratio is 1:1);
[0056] S2. Add a certain amount of deionized water to the mixture in step S1, and stir at room temperature;
[0057] S3, filtering the mixture in step S2, drying, and pulverizing;
[0058] S4, the product gained in step S3 is put into quartz boat and mixed with a certain proportion of sodium carbonate, then placed in tube furnace, under N 2 Under the atmosphere, pre-heat the tube furnace to 900-1000°C, then push the quartz boat to the heating center position, heat for 2-30min, and obtain square-shaped Fe at 900-980°C 4 (Fe(CN) 6 ) 3 Loaded carbon ribbons, 980~1000°C to obtain square FeOOH-loaded carbon ribbons;
[0059] S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com