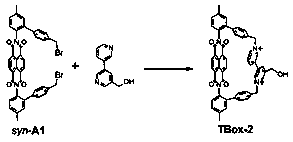Novel synthesis method of diimide macrocyclic compound
A technology based on macrocyclic compounds and imide groups, applied in the field of supramolecular chemistry, can solve the problems of difficult removal of templates, high dilution of solutions, cumbersome reaction operations, etc., and achieve good chemical properties of host and guest. The method is simple and time-consuming short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
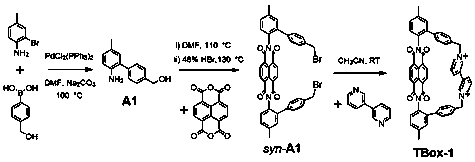
[0036] A kind of imide macrocyclic compound, the chemical formula of the macrocyclic compound is C 52 h 36 N 4 o 4 2+ , whose structure is:
[0037]
[0038] The synthetic method step of described macrocyclic compound is as follows:
[0039] (1) 2-bromo-4-methylaniline (7.00g, 37.63mmol), 4-hydroxymethylphenylboronic acid (6.86g, 45.16mmol), bis(triphenylphosphine)palladium dichloride (1.81g , 2.57mmol), sodium carbonate solution (75mL, 2M), DMF (115mL) were added into the round bottom flask, nitrogen protection ensured the reaction under anaerobic conditions, heated and stirred at 100°C for 4h, after the reaction was completed, water (230mL) was added Dilute, and then extract three times with ethyl acetate, the amount of ethyl acetate each time is 115mL, the organic phase is dried with anhydrous magnesium sulfate, after drying, the magnesium sulfate is removed by filtration, the filtrate is concentrated, and the solid powder A1 is separated by column chromatography 6....
Embodiment 2
[0044] A kind of imide macrocyclic compound, the chemical formula of the macrocyclic compound is C 53 h 38 N 4 o 5 2+ , whose structure is:
[0045]
[0046] The first three steps of the synthetic method of the macrocyclic compound are the same as in Example 1, and the fourth step is as follows: the intermediate product syn-A1 (204mg, 0.26mmol), (5-pyridin-3-ylpyridin-3-yl) Methanol (48mg, 0.26mmol) and acetonitrile (5mL) were added to a round-bottomed flask, under nitrogen protection to ensure the reaction under anaerobic conditions, stirred at 55°C for 6h, after the reaction, the obtained solid was dissolved in water (20mL), and saturated Ammonium hexafluorophosphate solution (20mL), precipitated, filtered, washed with water, dried to obtain solid powder, i.e. 154 mg of the target macrocyclic compound, the reaction yield was 55%, the macrocyclic compound NMR analysis, the data are as follows: 1 H NMR (600MHz, DMSO-d 6 ,298K): δ(ppm)=9.598(s,1H),9.483(s,1H),9.412(d,J...
Embodiment 3
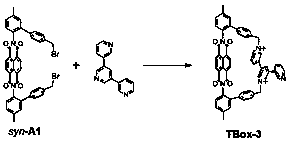
[0048] A kind of imide macrocyclic compound, the chemical formula of the macrocyclic compound is C 57 h 39 N 5 o 4 2+ , whose structure is:
[0049]
[0050] The first three steps of the synthetic method of described macrocyclic compound are identical with embodiment 1, and the 4th step is as follows:
[0051] Intermediate product syn-A1 (204mg, 0.26mmol), raw material 3,5-bis(pyridin-3-yl)pyridine (61mg, 0.26mmol), acetonitrile (5mL) were added in a round bottom flask, nitrogen protection ensured that there was no React under oxygen conditions, stir at 55°C for 8h, after the reaction, dissolve the obtained solid in water (20mL), add saturated ammonium hexafluorophosphate solution (20mL), a precipitate precipitates, filter, wash the precipitate with water, and dry to obtain a solid Powder, that is, 149 mg of the target macrocyclic compound, the reaction yield is 50%, and the nuclear magnetic analysis of the macrocyclic compound shows the following data: 1 H NMR (600MH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com