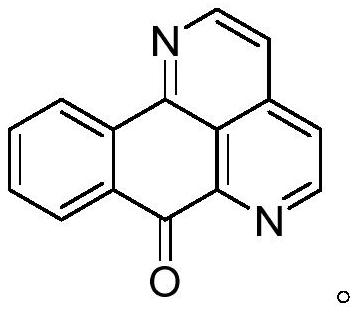
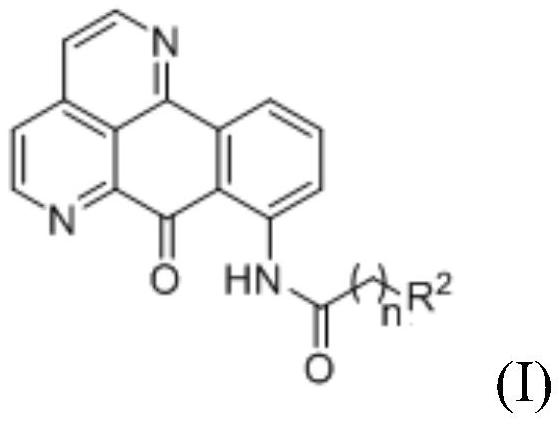
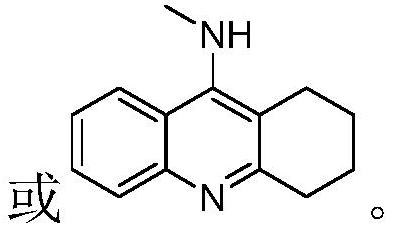
8-substituted 1,6-diazabenzanthrone derivatives and their synthesis methods and applications
A synthesis method and technology of benzanthrone, applied in the field of 8-substituted 1,6-diazabenzanthrone derivatives and their synthesis, to achieve good medicinal value and high blood-brain barrier permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Synthesis of 4-methyl-1-aza-5,10-anthraquinone (compound 3)
[0047] Under electromagnetic stirring, compound 1 (40.0g, 169.5mmol), xylene (500mL), and compound 2 (28.2g, 2.5mol) were successively added to a 1L round-bottomed flask, and refluxed for 6h (monitoring the reaction with TLC, developing Agent: V 乙酸乙酯 :V 石油醚 =1:1). After the reaction is completed, cool, transfer the product to an extraction bottle, extract with ethyl acetate (6×200ml), combine the organic phases, extract with 2N sulfuric acid (4×500ml), and obtain the acid layer solution Neutralize with 6N sodium hydroxide solution in an ice bath, adjust pH=9, filter with suction, and dry to obtain a crude product, which is purified by silica gel column chromatography (petroleum ether / ethyl acetate=100:1) to obtain compound 3 as gray Solid, yield 60%.
[0048] 1 H NMR (500MHz, Chloroform-d) δ: 8.88(d, J=4.8Hz, 1H), 8.37–8.31(m, 1H), 8.26~8.20(m, 1H), 7.85~7.76(m, 2H), 7.48(dd,J=4.8,0.9Hz,1H),2....
Embodiment 2
[0051] Example 2: Synthesis of 4-methyl-9-nitro-1-aza-5,10-anthraquinone (compound 4)
[0052] Under electromagnetic stirring, compound 3 (6.0 g, 26.9 mol) was added into a 100 mL round-bottomed flask, and concentrated sulfuric acid (25 mL) was slowly added dropwise under ice-bath conditions. After compound 3 was completely dissolved, fuming nitric acid ( 10mL); Reaction 24h (monitoring reaction with TLC, developer: V 乙酸乙酯 :V 石油醚 =2:1). After the reaction was completed, the mixture was slowly added to a beaker equipped with a small amount of ice water, neutralized by adding 6N sodium hydroxide solution dropwise in an ice bath, adjusted to pH=10, cooled, suction filtered, and dried. The crude product of compound 4 was obtained (the product can be directly subjected to the next step of reduction without purification).
Embodiment 3
[0053] Example 3: Synthesis of 4-methyl-9-amino-1-aza-5,10-anthraquinone (compound 5)
[0054] Under electromagnetic stirring, sequentially add the crude product of compound 4 (5.0 g), sodium sulfide nonahydrate (30.0 g), and absolute ethanol (250 mL) into a 500 mL round bottom flask, and reflux for 5 h (monitor the reaction with TLC, developing solvent: V 乙酸乙酯 :V 石油醚 =2:1). After the reaction was completed, cool, filter, and dry to obtain a crude product, which was purified by silica gel column chromatography (dichloromethane as the eluent) to obtain compound 5 as a red solid with a yield of about 70%.
[0055] 1 H NMR (400MHz, DMSO-d 6 )δ: 8.82(d, J=4.7Hz, 1H), 8.01(s, 2H), 7.60(d, J=4.6Hz, 1H), 7.50(t, J=7.8Hz, 1H), 7.30(d, J=7.2Hz, 1H), 7.17(d, J=8.4Hz, 1H), 2.75(s, 3H). 13 C NMR (100MHz, DMSO-d 6 )δ: 184.9, 182.29, 153.09, 151.9, 150.6, 149.9, 134.8, 134.3, 130.4, 128.1, 123.0, 115.3, 111.8, 22.1. HRMS (ESI) m / z: calcd for C 14 h 11 N 2 o 2 [M+H] + :239.0815, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com