Acrivastine novel crystal A and preparation method and application thereof
A crystal form and crystal technology, applied in the field of medicinal chemistry, can solve the problems of low chemical stability and achieve the effects of high chemical stability, high bioavailability, and easy process production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
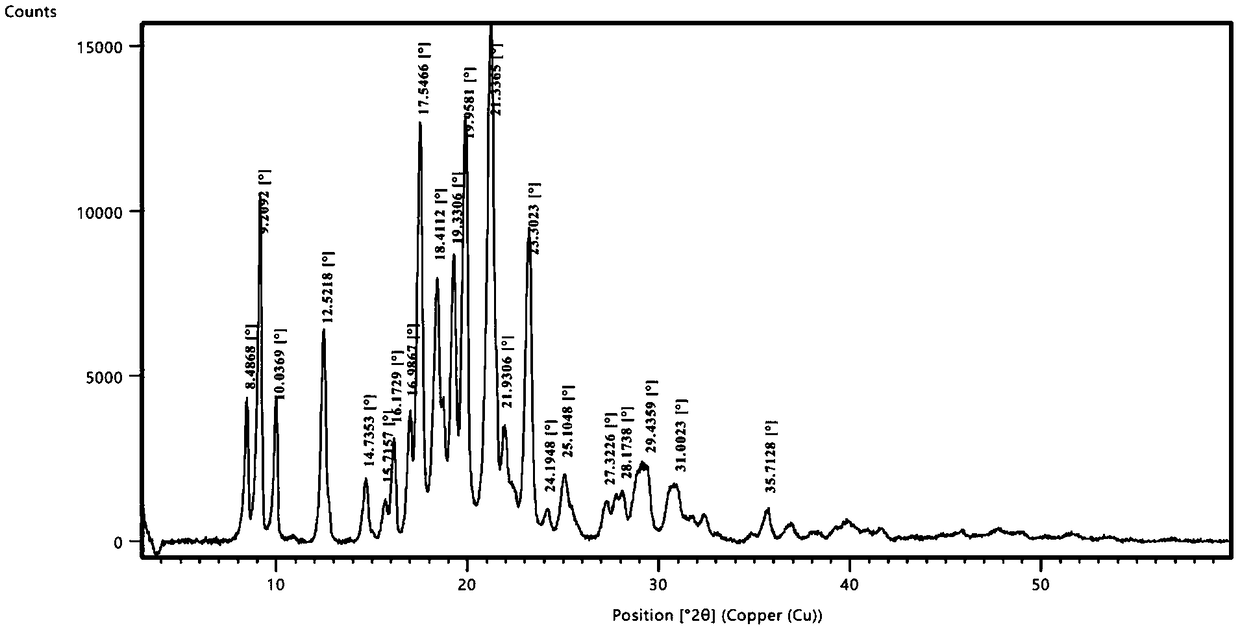
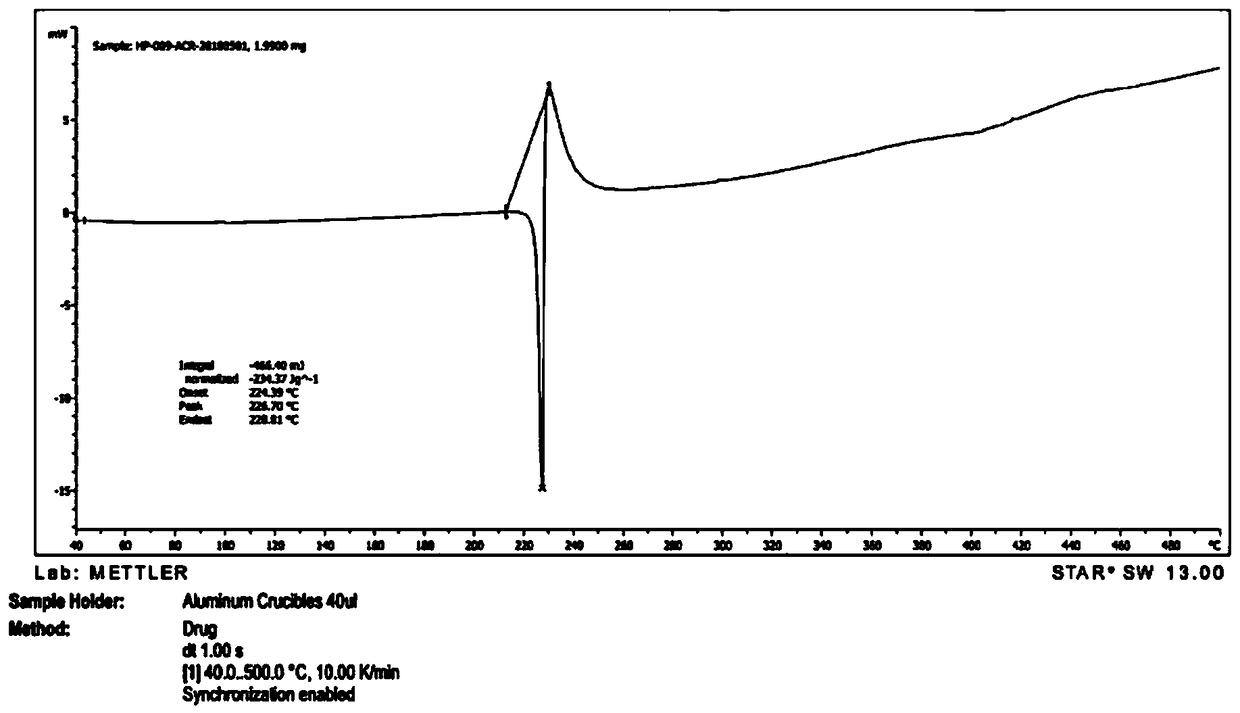
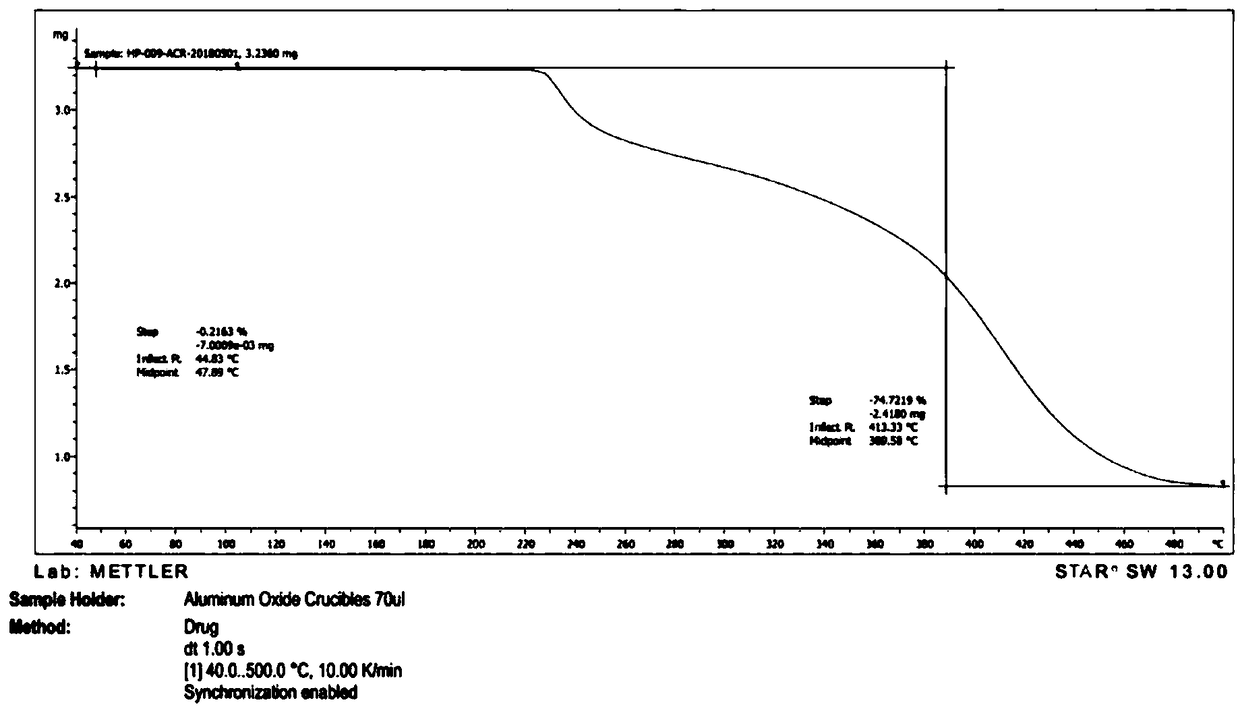
[0056] Take 2g of a sample of formula I and add it to 20ml of methanol, raise the temperature and reflux for 20 minutes, the system gradually dissolves, stir for 10 minutes, cool down to 10-15°C, a large amount of solids precipitate, stir for 30 minutes, filter, the filter cake is at 75-85°C, The vacuum pressure was -0.09Mpa~-0.10MPa and dried under reduced pressure for 5 hours to obtain 1.2g solid with a yield of 60%. It was confirmed to be crystal form A by X-ray powder diffraction. effect.
Embodiment 2
[0058] Take 2g of the sample of formula I and add it to 20ml of isopropanol, heat up and reflux for 20 minutes, if it is not dissolved, add 20ml of isopropanol, stir for 20 minutes, part of the solid dissolves, add 20ml of isopropanol, stir for 10 minutes, the system dissolves Clear, continue to stir for 10 minutes, cool down to 0-10°C and stir for 30 minutes, there is no solid precipitation, continue to cool to -15°C, there is solid precipitation, continue to stir for 30 minutes, filter, filter cake at 75-85°C, vacuum pressure Dry under reduced pressure at -0.09Mpa~-0.10MPa for 5 hours to obtain 1.6g of solid with a yield of 80%. It was confirmed to be crystal form A by X-ray powder diffraction. The preparation made of this material is equivalent to Xinminle Bio.
Embodiment 3
[0060] Take 200g of a sample of formula I and add it to 2800ml of ethanol, heat up to 75°C and stir, the system gradually dissolves, stir for 10 minutes, naturally cool down to 35-40°C, the system gradually becomes turbid, continue to cool down to 10-15°C, a large amount of solids precipitate , continue to cool down to -5~-15°C and stir for 1.5 hours, filter, filter cake at 75~85°C, vacuum pressure is -0.09Mpa~-0.10MPa and dry under reduced pressure for 8 hours to obtain 170g solid, yield 85%, after X -ray powder diffraction confirmed that it is crystal form A, and the preparation made of this raw material is equivalent to Xinminle Bio.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com