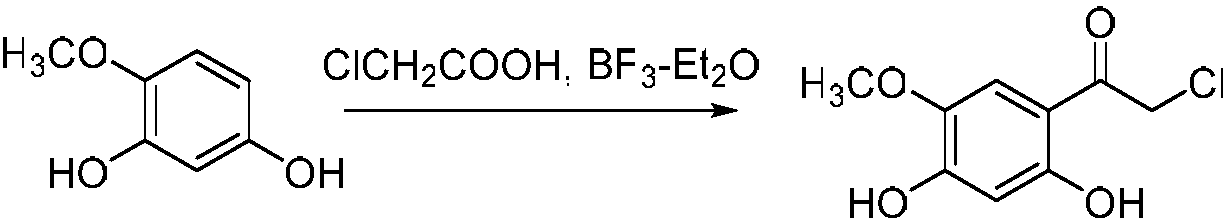Method for synthesizing glycitein
A synthesis method and glycidin technology are applied in the field of synthesis of glycidin and its analogs, can solve problems such as low yield, and achieve the effects of high yield, mild conditions and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Synthesis of 4-methoxy-6-chloroacetyl-1,3-benzenediol
[0021]
[0022] Dissolve 5g 4-methoxy 1,3-benzenediol in 100mL boron trifluoride ether solution, add 15g chloroacetic acid, react for 12h at 65℃, quench the reaction with 200mL ice saturated sodium bicarbonate solution, and extract with ethyl acetate (250mL × 3 times), the organic phases were combined, washed with saturated sodium chloride solution (200 mL × 3 times), dried with anhydrous sodium sulfate, filtered and concentrated, separated by silica gel column, V (petroleum ether): V (ethyl acetate) = 8 :1 eluted to obtain 5.77 g of white solid with a yield of 75.8%.
[0023] m.p.139-142℃; 1 H NMR(CDCl 3 ,400MHz)δ:3.92(s,3H,-OCH 3 ); 4.59(s,2H,-CH 2 Cl); 6.38(s,1H,3'-H); 6.57(s,1H,6'-H); 7.02(s,1H, 2'-OH); 12.06(s,1H,4'-OH) .
Embodiment 2
[0024] Example 2 Synthesis of 4-methoxy-6-bromoacetyl-1,3-benzenediol
[0025]
[0026] Under nitrogen protection, add 10mL dry redistilled CH to 380mg anhydrous aluminum trichloride (2.85mmol) 2 Cl 2 , 172.8mg bromoacetyl bromide (0.856mmol) was added dropwise under ice bath conditions, after stirring for 1 hour, 100mg 4-methoxy-1,3-benzenediol (0.714mmol) in CH 2 Cl 2 The solution was 10mL, reacted at 40℃ for 4h; quenched with ice water, extracted with dichloromethane (30mL×3 times), combined the organic phases, washed with saturated sodium chloride solution (20mL×3 times), dried with anhydrous sodium sulfate, filtered Concentrate, separate by silica gel column, and elute with V (petroleum ether): V (ethyl acetate) = 10:1 to obtain 60 mg of yellow needle crystals with a yield of 32.2%.
[0027] m.p.152-153℃; ESI-MS(m / z,%):261([M+H] + ,100); 1 H NMR (CDCl 3 ,400MHz)δ:3.92(s,3H,-OCH 3 ); 4.33(s,2H,-CH 2 Br); 6.39(s, 1H,3'-H); 6.54(s,1H,6'-H); 7.06(s,1H,2'-OH); 12.11(s,1H,4'-OH) .
Embodiment 3
[0028] Example 3 Synthesis of 3-chloro-6-methoxy-7-hydroxy-4H-chromen-4-one
[0029]
[0030] 730mg of 4-methoxy-6-chloroacetyl-1,3-benzenediol (3.37mmol) was dissolved in 5mL of dry redistilled DMF, 1mL of methanesulfonyl chloride was slowly added, and the reaction was carried out at 60°C for 2h. The reaction solution was poured into 50 mL of 5% sodium acetate solution, a solid was precipitated, and 655 mg of a white solid was obtained by filtration, with a yield of 85.8%.
[0031] m.p.207-218℃; ESI-MS m / z(%)227([M+H] + ,56); 229(M+3 + ,18); 1 H NMR(CDCl 3 ,400MHz)δ:4.02(s,3H,-OCH 3) ; 6.35 (s, 1H, 7-OH); 6.94 (s, 1H, 8-H); 7.60 (s, 1H, 5-H); 8.08 (s, 1H, 2-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com