Preparing method of anti-influenza virus preparation
A technology for anti-influenza virus and preparations, which is applied in the field of preparation of anti-influenza virus preparations, can solve the problems of rimantadine synthesis steps, environmental protection, long time, etc., and achieve cold symptoms relief, less by-products, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
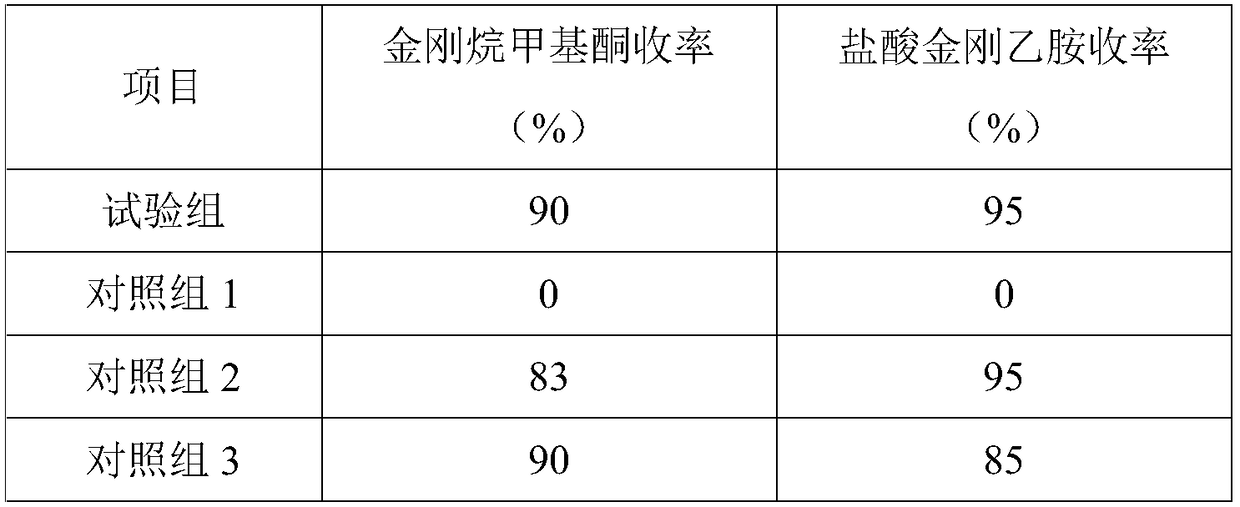
Examples
Embodiment 1
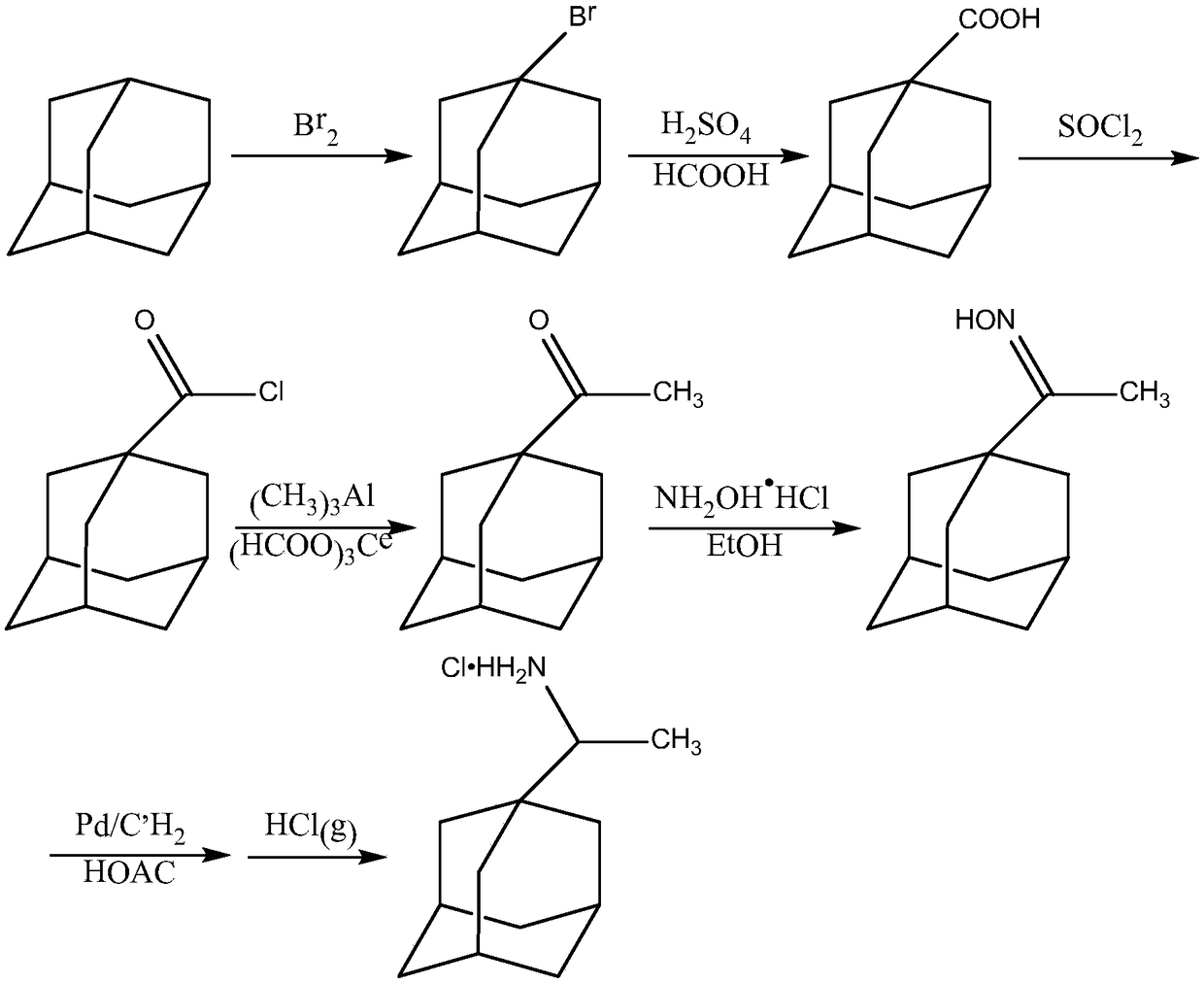
[0027] A preparation method of an anti-influenza virus preparation, comprising the preparation of 1-bromoadamantane, the preparation of adamantanecarboxylic acid, the preparation of adamantane carboxylic acid chloride, the preparation of adamantane methyl ketone, the preparation of 1-adamantane methyl ketone oxime, and the preparation of rimantadine hydrochloride Preparation, preparation of anti-influenza virus preparations, the specific steps include:
[0028] Preparation of 1-bromoadamantane: Add 30g of adamantane and 24mL of liquid bromine successively to the reaction flask, react at 85°C for 6h, then raise the temperature to 110°C, react in an oil bath for 3h, leave it overnight, recover the liquid bromine by distillation, and use 20 mL of saturated sodium bisulfite solution was used to reduce the remaining bromine, filtered, and the filter cake was washed with water until neutral, dried, and recrystallized from methanol to obtain 44 g of light yellow crystals with a yield ...
Embodiment 2
[0036] A preparation method of an anti-influenza virus preparation, comprising the preparation of 1-bromoadamantane, the preparation of adamantanecarboxylic acid, the preparation of adamantane carboxylic acid chloride, the preparation of adamantane methyl ketone, the preparation of 1-adamantane methyl ketone oxime, and the preparation of rimantadine hydrochloride Preparation, preparation of anti-influenza virus preparations, the specific steps include:
[0037] Preparation of 1-bromoadamantane: In the reaction flask, add adamantane and excess liquid bromine successively, react at 80°C for 6 hours, then raise the temperature to 120°C, react in an oil bath for 3 hours, leave it overnight, recover liquid bromine by distillation, and then use saturated Restore the remaining bromine with sodium bisulfate solution, filter, wash the filter cake with water until neutral, dry, and recrystallize from methanol to obtain light yellow crystals, which are 1-bromoadamantane;
[0038] Prepara...
Embodiment 3
[0045] A preparation method of an anti-influenza virus preparation, comprising the preparation of 1-bromoadamantane, the preparation of adamantanecarboxylic acid, the preparation of adamantane carboxylic acid chloride, the preparation of adamantane methyl ketone, the preparation of 1-adamantane methyl ketone oxime, and the preparation of rimantadine hydrochloride Preparation, preparation of anti-influenza virus preparations, the specific steps include:
[0046] Preparation of 1-bromoadamantane: In the reaction bottle, add adamantane and excess liquid bromine successively, react at 90°C for 7 hours, then raise the temperature to 120°C, react in an oil bath for 4 hours, leave it overnight, recover liquid bromine by distillation, and then use saturated Restore the remaining bromine with sodium bisulfate solution, filter, wash the filter cake with water until neutral, dry, and recrystallize from methanol to obtain light yellow crystals, which are 1-bromoadamantane;
[0047] Prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com