Method for preparing full color variable structural chromogenic material
A structural color-producing and variable technology, applied in chemical instruments and methods, fibrous fillers, inorganic pigment treatment, etc., can solve the problems that hinder the large-scale production, application, complex preparation process and slow color reaction speed of structural color-producing materials and other problems, to achieve high color saturation, simple preparation process, and save energy costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
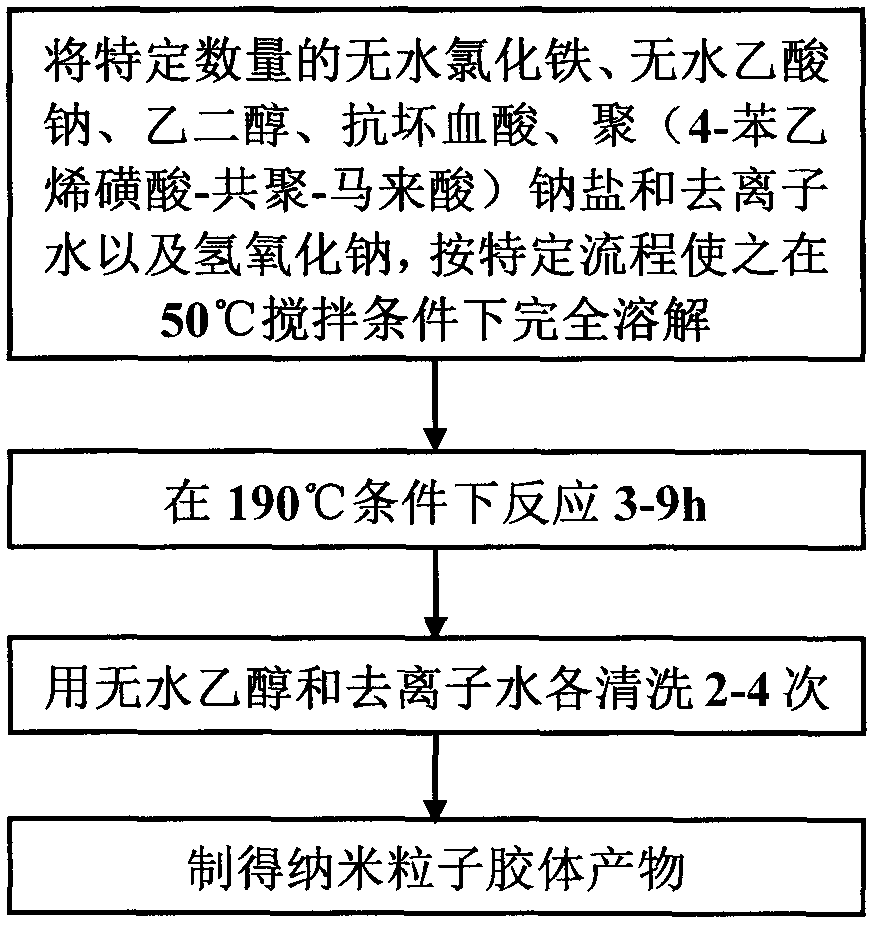
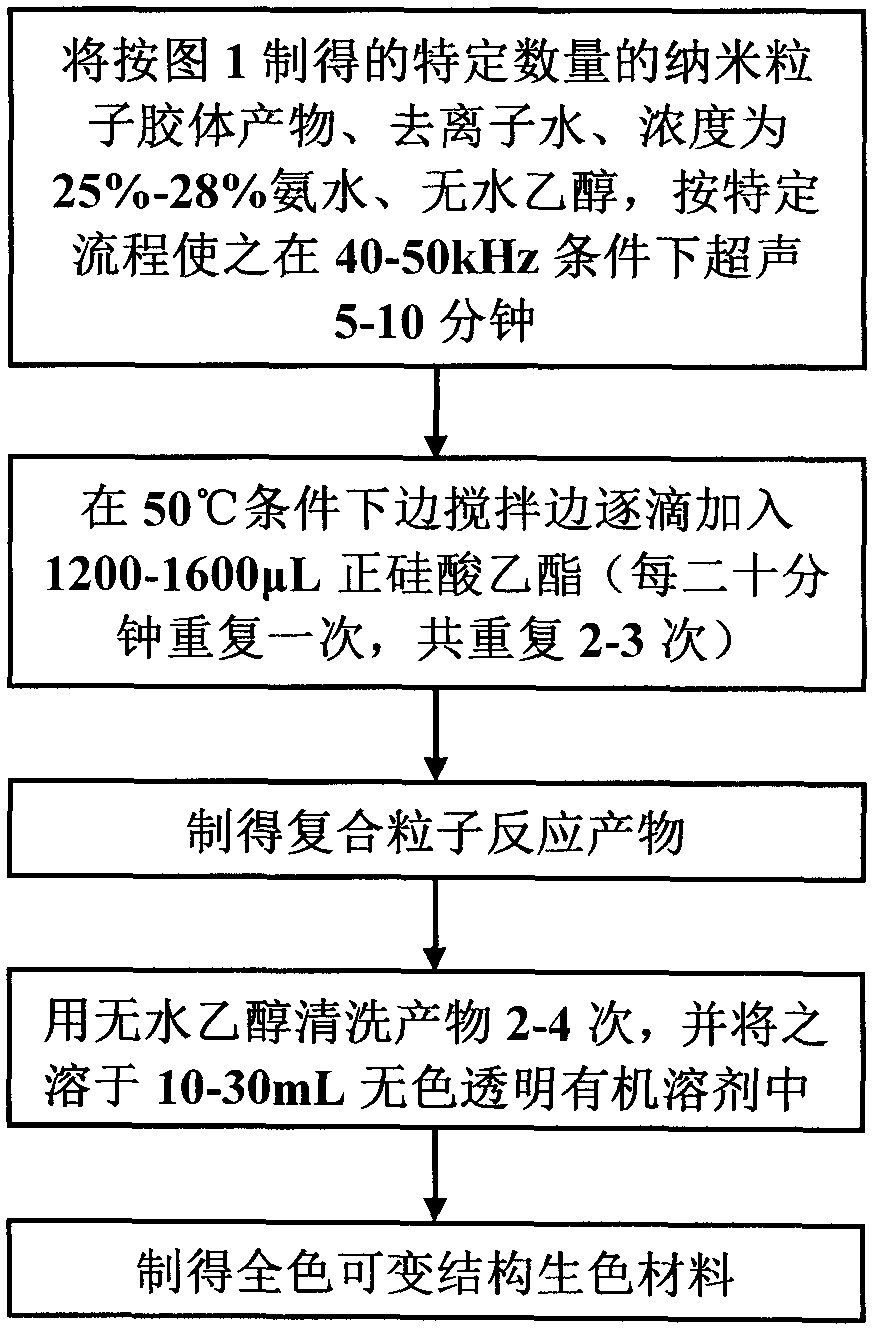
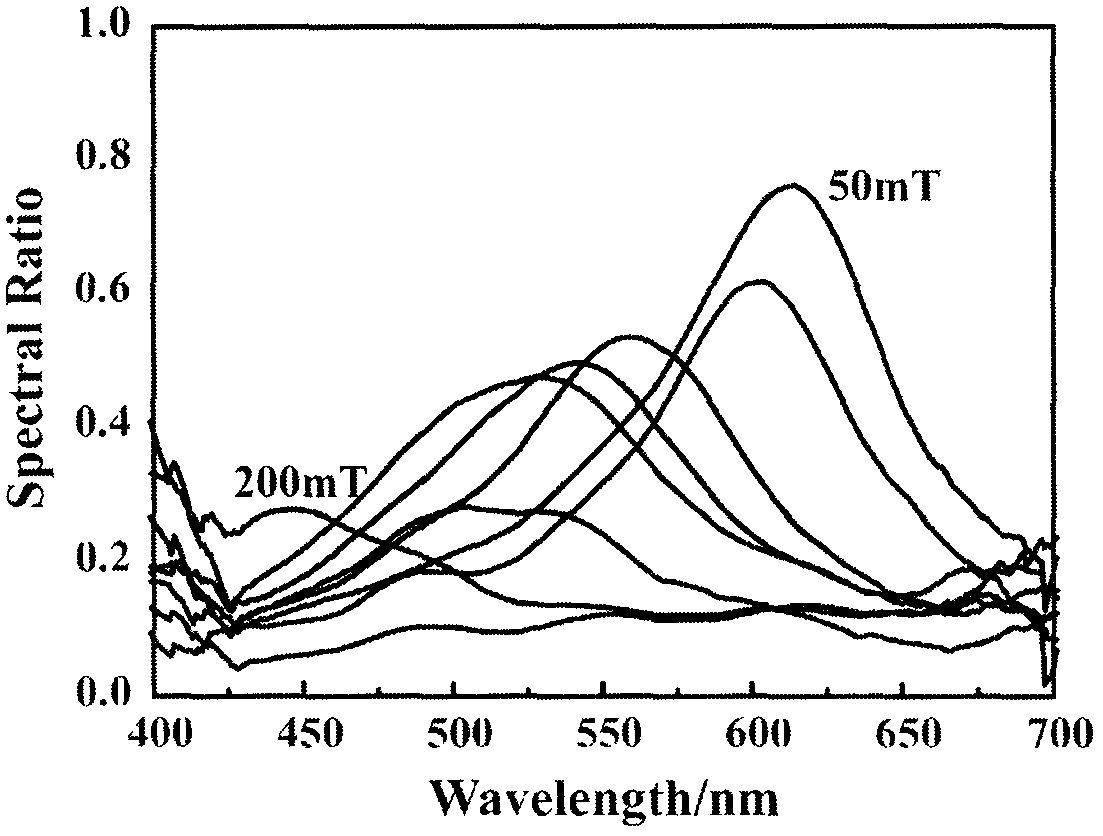
[0032]Step 1: Add 0.8g of anhydrous ferric chloride with a purity of 99.9% to 50mL of ethylene glycol, then add 3.5g of anhydrous sodium acetate, stir at 50°C to completely dissolve it, and obtain a light brown Yellow opaque solution; then add 0.012g of ascorbic acid and stir to make it completely dissolved to obtain a dark green opaque solution; then add 0.6g of poly(4-styrenesulfonic acid-copolymerization-maleic acid) sodium salt (3:1) Add 150 μL of deionized water and stir to completely dissolve it to obtain a dark yellow-brown opaque solution; then add 0.7 g of sodium hydroxide and stir to completely dissolve it to obtain a brown-red transparent solution, and place the above mixture at 190°C for reaction After 3 hours, the lower layer was dark black and the upper layer was transparent layered reaction product; the reaction product was washed twice with absolute ethanol and deionized water respectively to obtain the desired nanoparticle colloidal product. After testing, the...
Embodiment 2
[0036] Step 1: Add 0.9g of anhydrous ferric chloride with a purity of 99.9% to 55mL of ethylene glycol, then add 4.2g of anhydrous sodium acetate, stir at 50°C to completely dissolve it, and obtain a light brown Yellow opaque solution; then add 0.014g of ascorbic acid and stir to make it completely dissolved to obtain a dark green opaque solution; then add 1.4g of poly(4-styrenesulfonic acid-copolymerization-maleic acid) sodium salt (1:1) Add 175 μL of deionized water and stir to completely dissolve it to obtain a dark yellow-brown opaque solution; add 0.8 g of sodium hydroxide and stir to completely dissolve it to obtain a brown-red transparent solution, and place the above mixture at 190°C for reaction After 6 hours, the lower layer was dark black and the upper layer was transparent layered reaction product; the product obtained from the reaction was washed three times with absolute ethanol and deionized water respectively to obtain the desired colloidal product of nanopartic...
Embodiment 3
[0040] Step 1: Add 1.0g of anhydrous ferric chloride with a purity of 99.9% to 60mL of ethylene glycol, then add 5.0g of anhydrous sodium acetate, stir at 50°C to completely dissolve it, and obtain a light brown Yellow opaque solution; then add 0.018g of ascorbic acid and stir to make it completely dissolved to obtain a dark green opaque solution; then add 2.3g of poly(4-styrenesulfonic acid-copolymerization-maleic acid) sodium salt (1:1) Mix with 200 μL of deionized water and stir to dissolve completely to obtain a dark yellow-brown opaque solution; then add 1.0 g of sodium hydroxide and stir to completely dissolve to obtain a brown-red transparent solution, and place the above mixture at 190°C for reaction After 9 hours, the lower layer was dark black and the upper layer was transparent layered reaction product; the reaction product was washed 4 times with absolute ethanol and deionized water respectively to obtain the desired nanoparticle colloidal product. After testing, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com