Preparation method of lithium bis(trifluoromethanesulphonyl)imide salt
A technology of trifluoromethylsulfonyl and lithium bisfluorosulfonimide is applied in the field of preparation of diimide lithium salt, which can solve the problems of difficult separation of by-products, high price, difficult operation and the like, so as to improve product performance and cost competitiveness, reduce adverse effects, and reduce the effect of separation difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
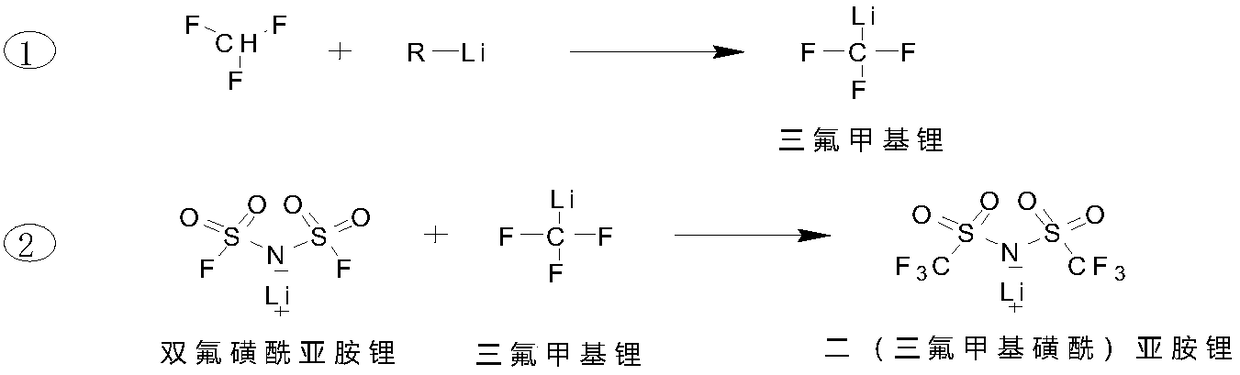
[0030] First, cool 3L (1.5mol) of 0.5mol ethyllithium n-butyl ether solution to -70°C~-50°C, start to feed trifluoromethane gas, adjust the gas flow according to the reaction temperature, and control the reaction temperature at -70°C~- 50°C, stop the ventilation when the reaction temperature does not change significantly, the reaction time is 3 hours, and the trifluoromethyllithium ether solution is obtained. The synthesis of the reaction solution is complete and it is ready for use; In n-butyl ether, prepare a lithium bisfluorosulfonyl imide solution, then slowly add the solution dropwise to the synthesized trifluoromethyllithium n-butyl ether solution, and control the reaction temperature between -30°C and 0°C During the dropwise addition process, white lithium fluoride solids will be precipitated, and after the lithium bisfluorosulfonimide solution is added dropwise, the reaction will end for two hours; the reaction solution is filtered, and the obtained filtrate is concentr...
Embodiment 2
[0032] First, mix 2.5 mol n-butyllithium hexane solution 2L (4.5 mol) with diethyl ether (2L) and cool to -70°C ~ -50°C, start to feed trifluoromethane gas, adjust the gas flow rate according to the reaction temperature, the reaction temperature Control the temperature at -70°C to -50°C, and stop ventilation when the reaction temperature has no obvious change. The reaction time is 5 hours, and the synthesis of the trifluoromethyllithium reaction solution is completed and is ready for use. Then 374 g (2.0 mol) of lithium bisfluorosulfonimide was dissolved in ether to prepare a lithium bisfluorosulfonyl imide ether solution, and then the solution was slowly added dropwise to the synthesized lithium trifluoromethyl ether solution , control the reaction temperature between -30°C and 0°C, white lithium fluoride solids will precipitate during the dropwise addition, and the reaction will end after two hours of adding the lithium bisfluorosulfonyl imide solution dropwise. The reaction...
Embodiment 3
[0034] First, mix 1L (2.0mol) of 2mol tert-butyllithium pentane solution with tert-butyl ether and cool it to -70°C~-50°C, then start to feed trifluoromethane gas, adjust the gas flow rate according to the reaction temperature, and control the reaction temperature At -70°C to -50°C, stop ventilation when there is no significant change in the reaction temperature, the reaction time is 3 hours, the synthesis of the trifluoromethyllithium reaction solution is completed, and it is ready for use; first, 178g of lithium bisfluorosulfonyl imide (0.95 mol) was dissolved in n-butyl ether to prepare lithium bisfluorosulfonimide solution, and then slowly added the solution dropwise to the synthesized trifluoromethyllithium n-butyl ether solution, controlling the reaction temperature at -30°C to Between 0°C, white lithium fluoride solids will precipitate during the dropping process, and the reaction will end after two hours of adding the lithium bisfluorosulfonyl imide solution dropwise. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com