Soluble resin substituted anthryl deep blue-light emitting material and preparation and application thereof
A blue-light material and soluble technology, which is applied in the direction of luminescent materials, semiconductor/solid-state device manufacturing, electrical components, etc., can solve the problems of lower efficiency and easy quenching of luminescence, and achieve inhibition of aggregation, good film shape stability, and good heat dissipation. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
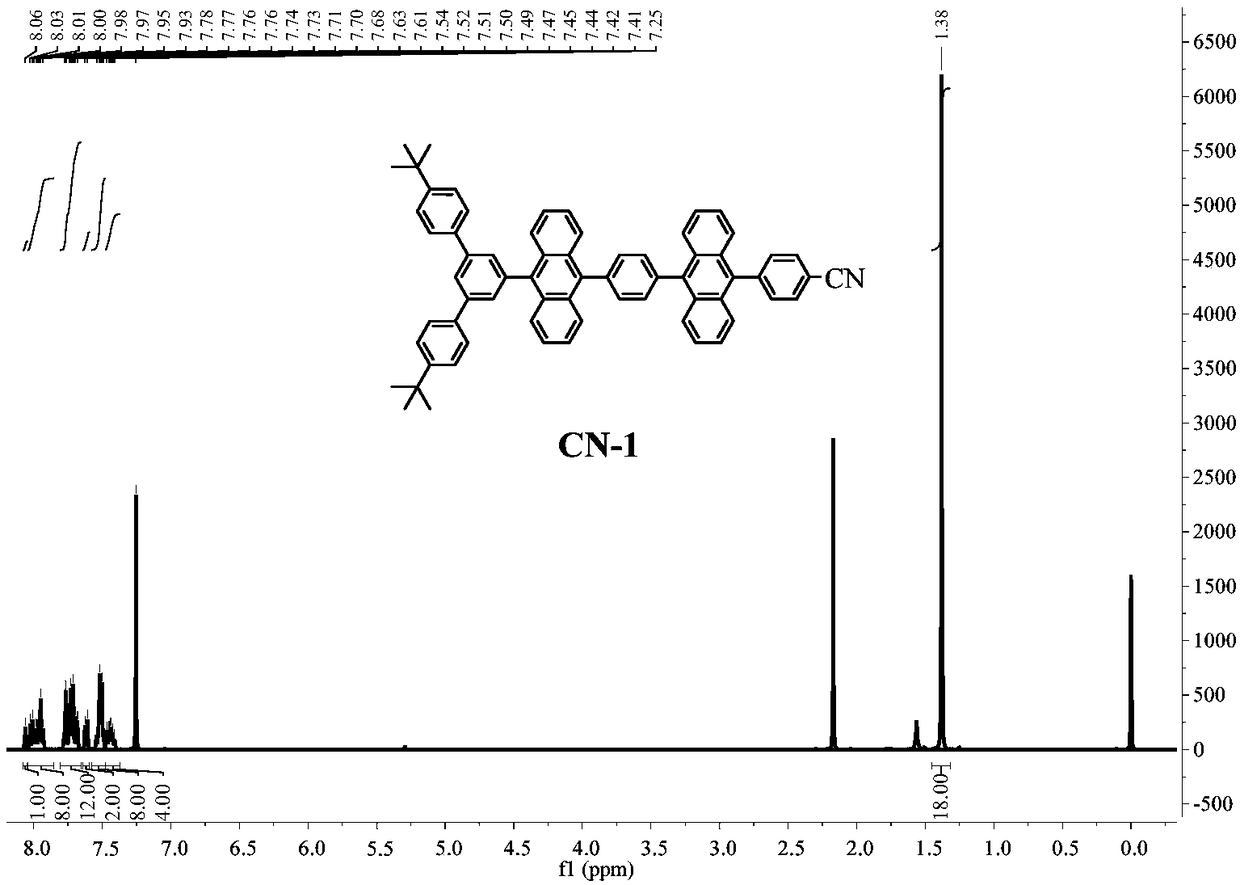
[0047] Example 1, anthracenyl blue light material CN-1
[0048] An anthracenyl TTA blue light material CN-1[4-(10-(4-(10-(4,4"-bis-tert-butyl-[1,1':3',1"-triphenyl]- 5'-yl) anthracene-9-yl) phenyl) anthracene-9-yl) benzonitrile], its structure is as follows:
[0049]
[0050] The preparation method of the anthracenyl blue light material CN-1 comprises the following steps:
[0051] Step 1, the preparation of 4-(anthracene-9-yl)benzonitrile:
[0052]
[0053] Under nitrogen atmosphere, 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrile (10.7g, 46.7mmol), 9 -Bromoanthracene (12.6g, 49mmol), potassium carbonate aqueous solution (30mL, 2M) was added to tetrahydrofuran (150mL), stirred for 20min, and tetrakis(triphenylphosphine) palladium (180mg, 0.155mmol) was added, overnight at 70°C Reaction, tetrahydrofuran was distilled off under reduced pressure, then extracted and dried, separated and purified by silica gel column to obtain a golden yellow solid (eluent: PE / ...
Embodiment 2
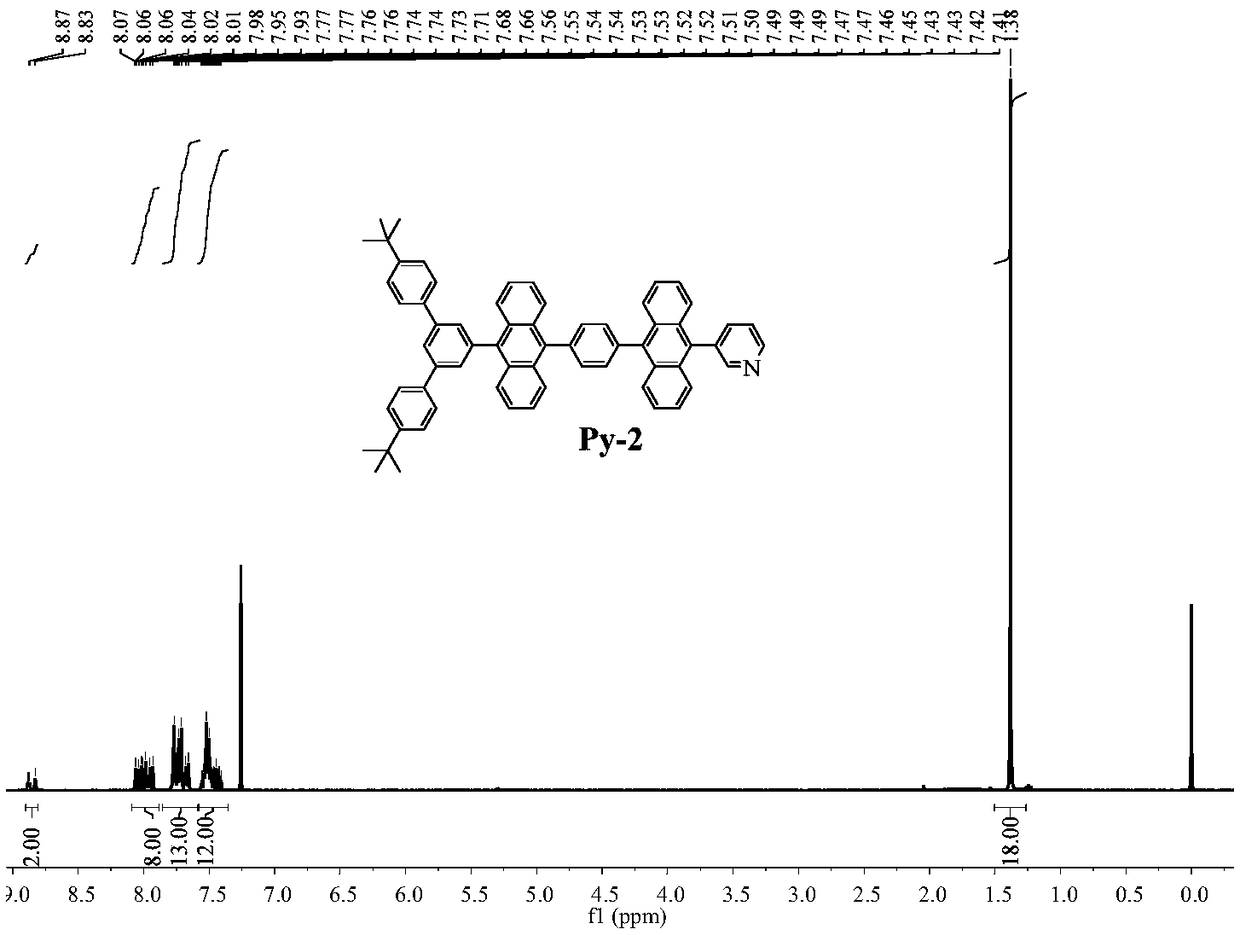
[0071] Example 2, anthracenyl blue light material Py-2
[0072] An anthracenyl blue light material Py-2[3-(10-(4-(10-(4,4"-di-tert-butyl-[1,1':3',1"-triphenyl]- 5'-yl) anthracene-9-yl) phenyl) anthracene-9-yl) pyridine], its structure is as follows:
[0073]
[0074] The preparation method of the anthracenyl blue light material Py-2 comprises the following steps:
[0075] Step 1, the preparation of 3-(anthracene-9-yl)pyridine:
[0076]
[0077] Under nitrogen atmosphere, pyridine-3-boronic acid (2.5g, 20mmol), 9-bromoanthracene (8.5g, 24mmol), potassium carbonate solution (20mL, 2M), ethanol (10mL) were added to toluene (50mL), After 20min, tetrakis(triphenylphosphine)palladium (160mg, 0.14mmol) was added and heated to reflux for 12h. After the reaction, the toluene was distilled off under reduced pressure, extracted and dried, and purified by silica gel column separation (PE / DCM). 2 g of white solid were obtained (yield 51%).
[0078] Step 2, the preparation of 3-(10-...
Embodiment 3
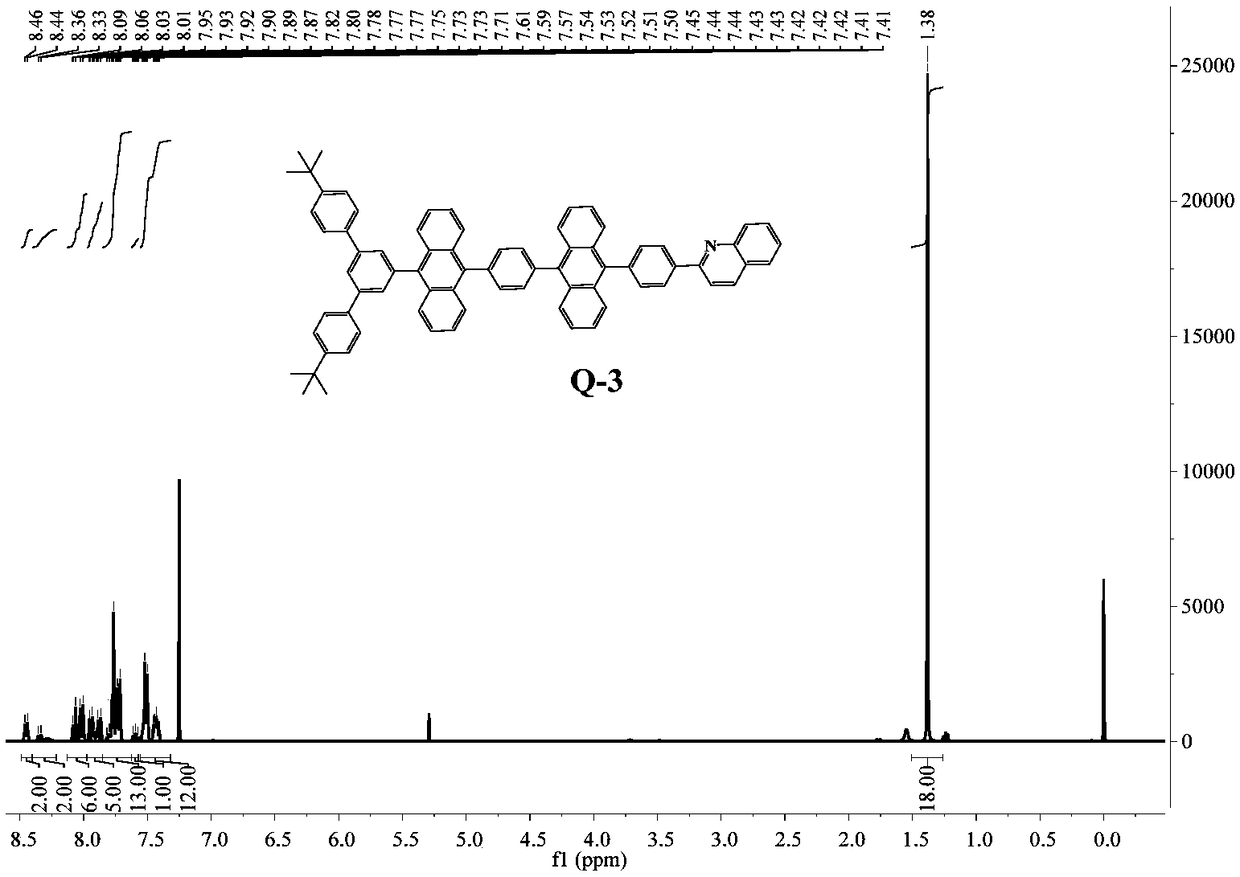
[0086] Example 3, anthracenyl blue light material Q-3
[0087] An anthracenyl blue light material Q-3[2-(4-(10-(4-(10-(4,4"-di-tert-butyl-[1,1':3',1"-terphenyl ]-5'-yl) anthracene-9-yl) phenyl) anthracene-9-yl) phenyl) quinoline], its structure is as follows:
[0088]
[0089] The preparation method of the anthracenyl blue light material Q-3 comprises the following steps:
[0090] Step 1, the preparation of 2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)quinoline:
[0091]
[0092] Under nitrogen atmosphere, dissolve 2-(4-bromophenyl)quinoline (3.5g, 12.3mmol), bis-pinacol borate (4.7g, 18.5mmol), potassium acetate (3.6g, 36mmol) In 50mL tetrahydrofuran, after degassing for 20min, add bis(triphenylphosphine)palladium dichloride (100mg, 0.142mmol), and heat to reflux overnight for reaction. Recrystallization afforded 3 g of product (73% yield).
[0093] Proton NMR spectrum analysis results: 1 H NMR (500MHz, CDCl 3 )δ8.26-8.15 (m, 4H), 7.97 (d, J = 8.2Hz, 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com