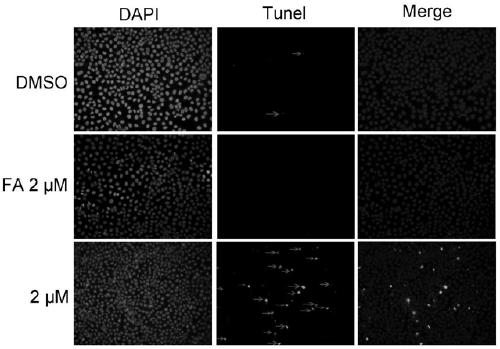
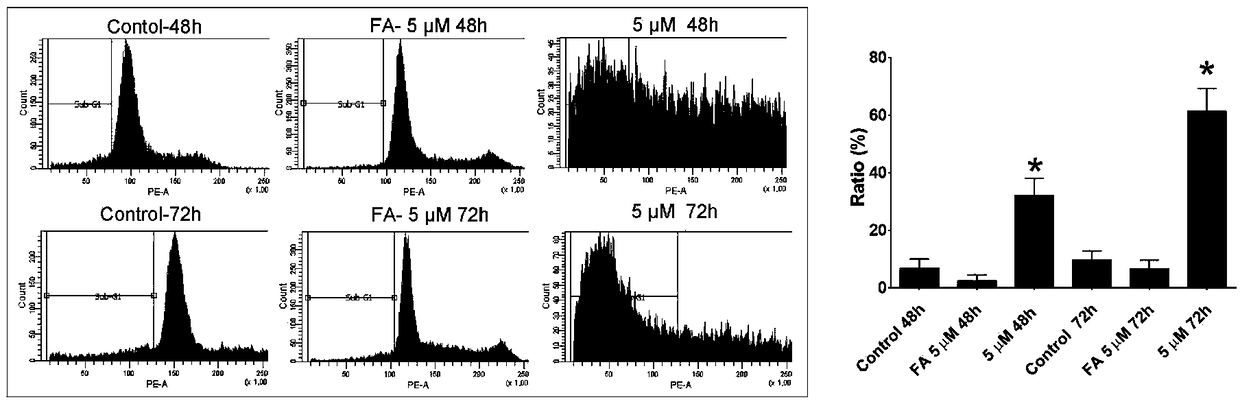
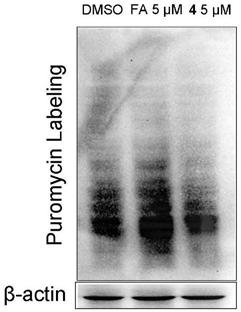
Fusidic acid derivative with anti-tumor activity and synthesis and preparation method thereof
A technology of fusidic acid and derivatives, applied in the field of fusidic acid derivatives, can solve problems such as no anti-tumor activity, and achieve the effect of anti-tumor mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 3β-[(2-Amino)acetoxy]-21-fusidic acid benzyl ester
[0048] Take a 500mL eggplant-shaped bottle, dissolve fusidic acid (10.01g, 0.019mol) in acetone (200mL), stir and add potassium carbonate (5.36g, 0.039mol), benzyl bromide (2.78mL, 0.023mol), 30°C React for 5-7 hours. Suction filtration, concentration, dilution with ethyl acetate (50mL), washing with 10% hydrochloric acid until acidic, washing with water, washing with saturated brine, drying over anhydrous sodium sulfate, filtering, distilling off the solvent under reduced pressure, silica gel column chromatography (V 氯仿 :V 甲醇 =210:1-190:1), to obtain benzyl 21-fusidate (8.86 g, 75.4%) as a white solid.
[0049] Take a 25mL eggplant-shaped bottle, dissolve benzyl 21-fusidate (0.10mmol) in anhydrous dichloromethane (10mL), stir and add Boc-protected amino acid (0.22mmol), DMAP (0.31mmol), EDCI ( 0.30mmol), react at room temperature for 4-6 hours. Wash with 10% hydrochloric acid until acidic, wash with water, wash w...
Embodiment 2
[0052] 3β-[(2-amino)propionyloxy]-21-fusidic acid benzyl ester
[0053] Using X2 as raw material, referring to the preparation method of benzyl 3β-[(2-amino)acetoxy]-21-fusidate, a white solid (46 mg, 86.7%) was obtained. 1 H-NMR (CDCl 3 ,400MHz)δ:7.29-7.35(m,5H,5×Ar-H),5.88(d,J=8.27Hz,1H,16-H),5.20(d,J=12.18Hz,1H,CHAr), 5.05(t, J=7.16Hz, 1H, 24-H), 4.91(d, J=2.46Hz, 1H, 11-OH), 4.92(d, J=12.14Hz, 1H, CHAr), 4.64(d, J=24.20Hz, 2H, -NH 2 ),4.31(s,1H,11-H),3.59(q,J=6.38Hz,1H,-CH-),3.01(d,J=11.13Hz,1H,13-H),2.38-2.49(m ,2H,2×22-H),2.24-2.28(m,1H,12-H),2.05-2.19(m,5H,1-H,5-H,15-H and 2×23-H), 1.92(s,3H,OCOCH 3 ),1.67-1.89(m,4H,2×2-H,7-H and 12-H),1.62(s,3H,27-CH 3 ),1.54-1.60(m,3H,1-H,6-H and 9-H),1.51(s,3H,26-CH 3 ),1.47(s,1H,4-H),1.36(s,3H,-CH 3 ),1.35(s,3H,30-CH 3 ),1.24-1.31(m,1H,15-H),1.09-1.17(m,2H,6-H and 7-H),0.97(s,3H,19-CH 3 ),0.91(s,3H,18-CH 3 ),0.83(d,J=6.69Hz,3H,28-CH 3 ).
Embodiment 3
[0055] Benzyl 3β-[(2-amino-4-methyl)pentanoyloxy]-21-fusidate
[0056] Using X3 as raw material, referring to the preparation method of benzyl 3β-[(2-amino)acetoxy]-21-fusidate, a white solid (28 mg, 79.4%) was obtained. 1 H-NMR (CDCl 3 ,400MHz)δ:7.30-7.36(m,5H,5×Ar-H),5.88(d,J=8.26Hz,1H,16-H),5.20(d,J=12.21Hz,1H,CHAr), 5.06(t, J=6.94Hz, 1H, 24-H), 4.91-4.94(m, 2H, CHAr and 11-OH), 4.64(d, J=23.61Hz, 2H, -NH 2 ), 4.31(s, 1H, 11-H), 3.49(t, J=7.43Hz, 1H, -CH-), 3.02(d, J=11.58Hz, 1H, 13-H), 2.38-2.52(m ,2H,2×22-H),2.26-2.29(m,1H,12-H),2.07-2.20(m,3H,15-H and 2×23-H),1.96-2.04(m,2H, 1-H and 5-H),1.92(s,3H,OCOCH 3 ),1.68-1.88(m,4H,2×2-H,7-H and 12-H),1.63(s,3H,27-CH 3 ),1.54-1.61(m,3H,1-H,6-H and 9-H),1.52(s,3H,26-CH 3 ),1.38-1.46(m,1H,4-H),1.36(s,3H,30-CH 3 ),1.25-1.33(m,1H,15-H),1.03-1.17(m,2H,6-H and 7-H),0.98(s,3H,19-CH 3 ),0.94(s,3H,-CH 3 ), 0.93 (d, J=1.78Hz, 3H, 18-CH 3 ),0.91(s,3H,-CH 3 ),0.83(d,J=6.66Hz,3H,28-CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com