Sterile rapid detection system of medical equipment and application method thereof
A technology for medical devices and detectors, applied in the field of medical device sterility rapid inspection systems, can solve the problems of strong subjectivity, difficult judgment, false positives, etc., to reduce subjective judgment errors, shorten the detection period, and reduce the use time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Such as figure 1 , 2 shown.
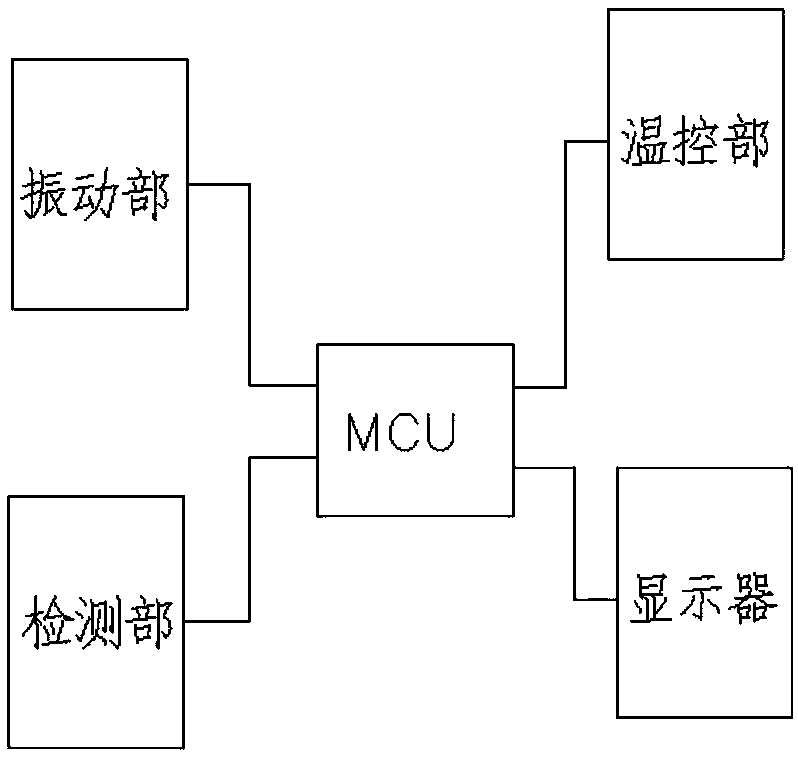
[0055] A medical device sterility rapid inspection system, comprising: an automatic tester for sterility test and a culture bottle, the culture bottle is used to contain culture medium and sterilized biological indicator bacteria, and the culture bottle is used to be placed in the sterility test In the automatic detector, the automatic detector for sterility test carries out vibrating culture to the culture bottle;
[0056] The sterility test automatic detector also includes a vibration part, a temperature control part, and a detection part; the vibration part, the temperature control part and the detection part are connected to the MCU chip respectively;
[0057] The vibrating part is used for vibrating the culture bottle;
[0058] The temperature control part is used to control the temperature of cultivation;
[0059] The detection part is used for real-time absorbance detection, pH value detection,
[0060] Metabolic end product CO ...
Embodiment 2
[0068] Such as Figure 4 , 5 shown.
[0069] The culture bottle suitable for the system described in embodiment 1 includes a transparent bottle body 1, a bottle cap 2 is arranged on the upper end of the transparent bottle body 1, and the bottle cap 2 and the transparent bottle body 1 are connected by threads; The bottom of the bottle body 1 is provided with a silicone membrane ring 3, the cross section of the silicone membrane ring 3 is a semi-closed shape, and the silicone membrane ring and the bottom of the transparent bottle form a closed annular area. A detection reagent 4 is set in the area.
[0070] The silica gel diaphragm is a polymer semipermeable membrane. The polymer semipermeable membrane allows CO 2 permeable membrane, and the detection reagent is a pH value chromogenic method value chromogenic reagent. CO 2 Permeable through the silica gel membrane, when microorganisms grow in the culture bottle, the released CO 2 After permeating to the silica gel membran...
Embodiment 3
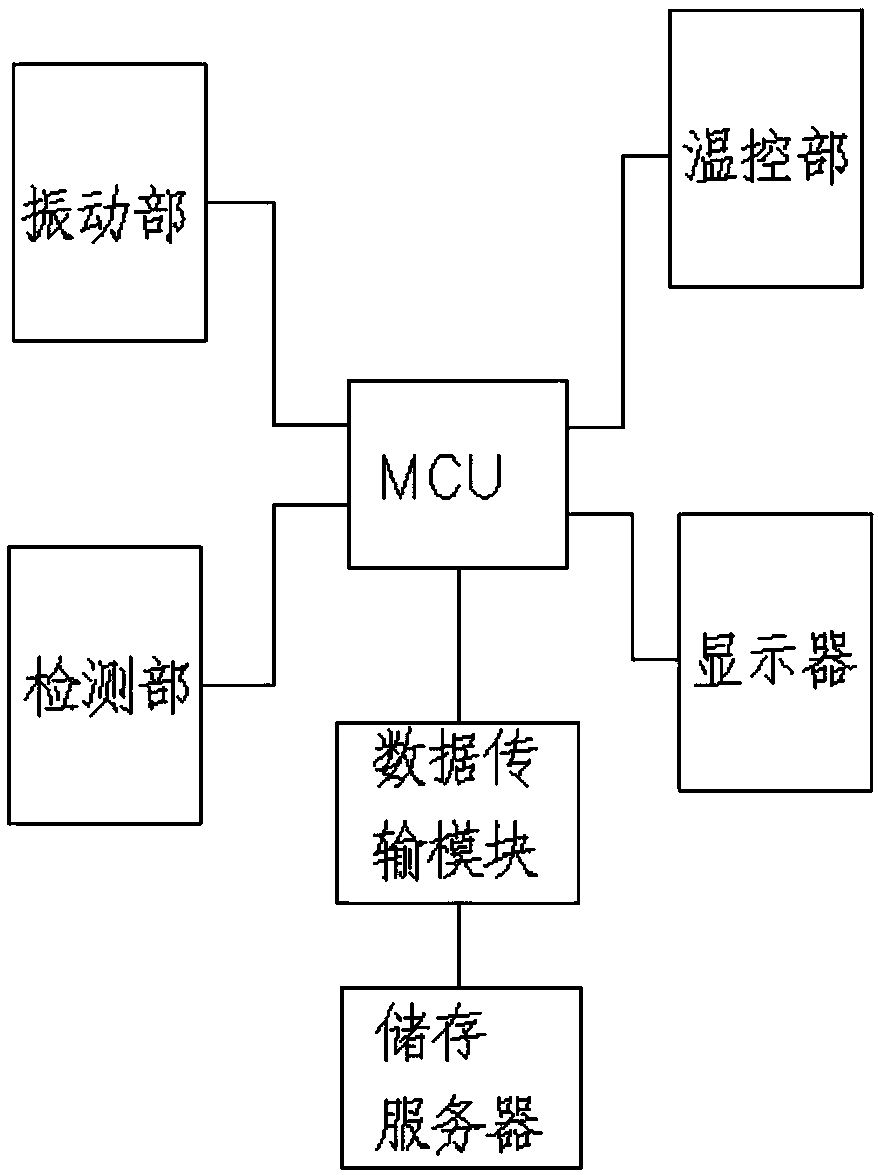
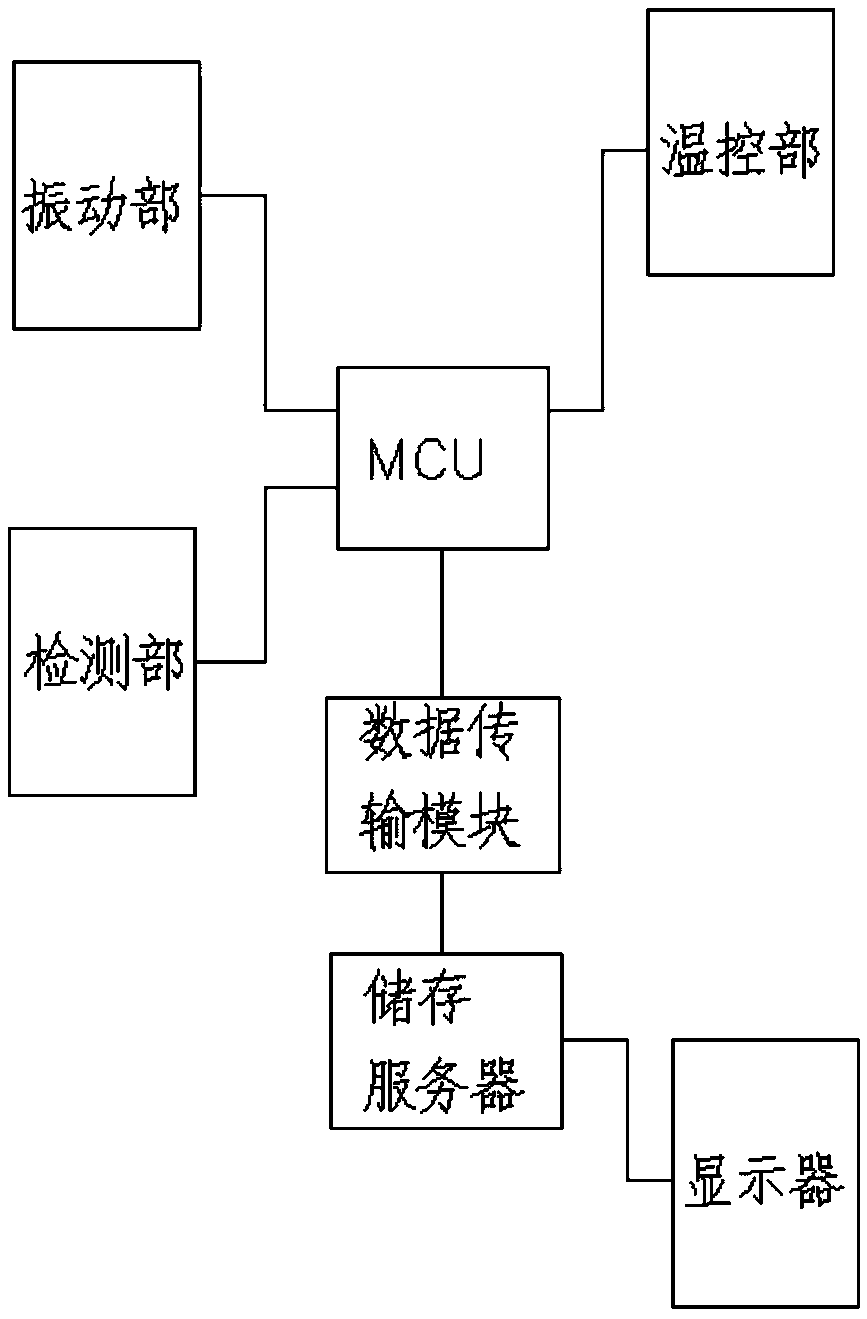
[0075] Such as image 3 shown.
[0076] A medical device aseptic rapid inspection system as described in Example 1, the difference is that the system also includes a data transmission module: used to collect various types of sterility inspection data in real time, and transmit the inspection data to the outside of the system The storage server; the system also includes a display, the display is used to display the test data or the experimental chart formed corresponding to the processed test data, and the display is connected to the storage server.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com