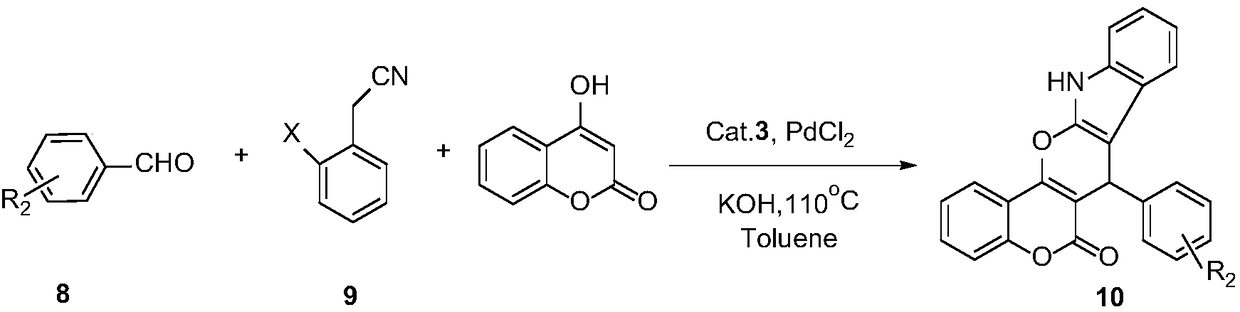
Preparation and application of 1,7-disubstituted aminomethyl-2,8-dyhydroxy-Troger's Base catalyst
A -base, aminomethyl technology, applied in the field of organic synthesis, can solve problems such as no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
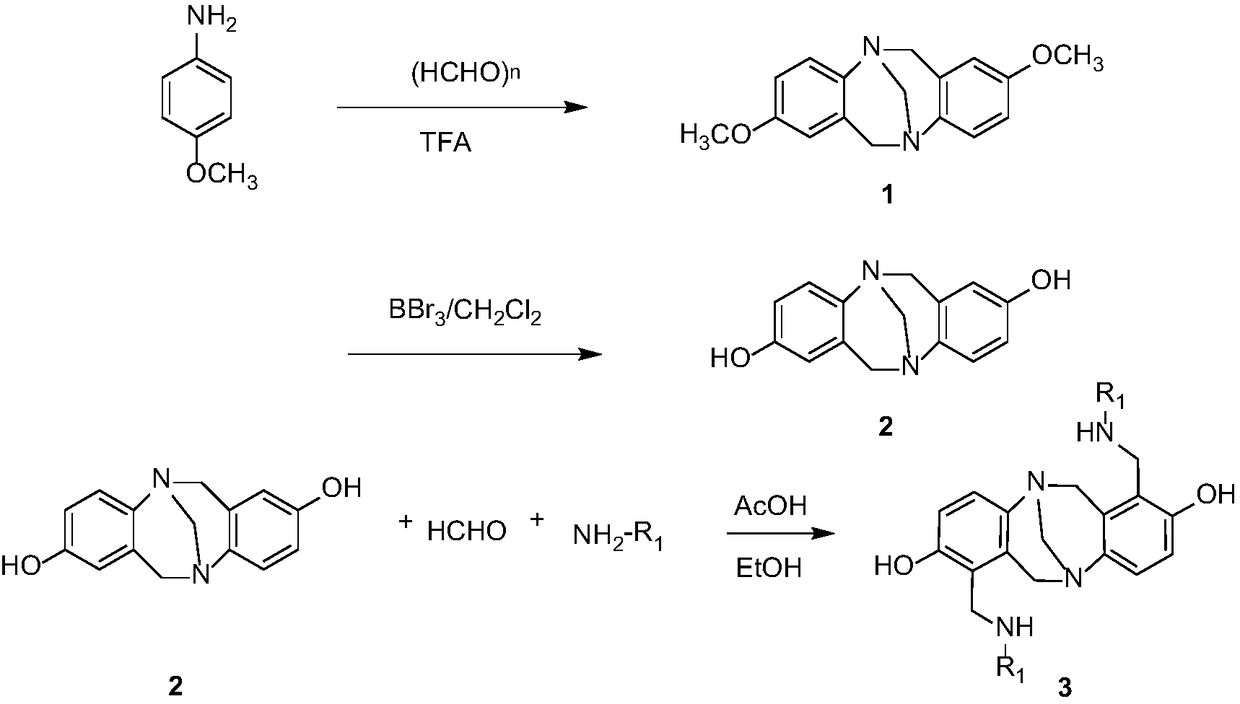
[0042] Embodiment 1: preparation compound 3a
[0043] Add formaldehyde (1.2mmol), cyclohexylamine (1.2mmol), glacial acetic acid (1mol%) and ethanol (3mL) successively into a 50mL dry round-bottomed flask. mmol), followed by TLC until the reaction was complete, the excess solvent was evaporated under reduced pressure, and recrystallized (EtOH) to obtain compound 3a with a yield of 83%.
[0044] The structural formula of compound 3a is:
[0045] The molecular formula is: C 29 h 40 N 4 o 2
[0046]The Chinese name is: 1,7-bis((cyclohexylamino)methyl)-6H, 12H--5,11-methanodibenzo[b,f] [1,5] diazocine-2,8- diol
[0047] The English name is:
[0048] 1,7-Bis((cyclohexylamino)methyl)-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine-2,8-diol
[0049] Appearance: white solid
[0050] Melting point: 153.5-154.2°C
[0051] Proton NMR spectrum: 1 H NMR (400MHz, CDCl 3 )δ6.95(d, J=8.8Hz, 2H), 6.66(d, J=8.8Hz, 2H), 4.89(d, J=9.4Hz, 1H), 4.81(d, J=10.0Hz, 1H) ,4.44(d,J=16.6Hz,2...
Embodiment 2
[0054] Embodiment 2: preparation compound 3b
[0055] Add formaldehyde (1.2mmol), methylamine (1.2mmol), glacial acetic acid (1mol%) and ethanol (3mL) successively into a 50mL dry round bottom flask, and after reflux reaction at 80°C for 2h, add intermediate 2 (1.0mmol ), followed by TLC until the reaction was complete, the excess solvent was evaporated under reduced pressure, and recrystallized (EtOH) to obtain compound 3b with a yield of 87%.
[0056] The structural formula of compound 3b is:
[0057] The molecular formula is: C 19 h 24 N 4 o 2
[0058] The Chinese name is: 1,7-bis((methylamino)methyl)-6H, 12H--5,11-methanodibenzo [1,5] diazocine-2,8- diol
[0059] The English name is:
[0060] 1,7-Bis((methylamino)methyl)-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine-2,8-diol
[0061] Appearance: white solid
[0062] Melting point: 244.3-247.1°C
[0063] Proton NMR spectrum: 1 H NMR (400MHz, CDCl3) δ6.97(d, J=8.8Hz, 2H), 6.71(d, J=8.8Hz, 2H), 4.67(s, 4H), 4.42...
Embodiment 3
[0066] Embodiment 3: preparation compound 3c
[0067] Formaldehyde (1.2mmol), n-butylamine (1.2mmol), glacial acetic acid (1mol%) and ethanol (3mL) were added successively to a 50mL dry round bottom flask, and intermediate 2 (1.0 mmol), followed by TLC until the reaction was complete, the excess solvent was evaporated under reduced pressure, and recrystallized (EtOH) to obtain compound 3c with a yield of 85%.
[0068] The structural formula of compound 3c is:
[0069] The molecular formula is: C 25 h 36 N 4 o 2
[0070] Chinese name: 1,7-bis((butylamino)methyl)-6H, 12H--5,11-methanodibenzo [b, f] [1,5] diazocine-2,8- diol
[0071] The English name is:
[0072] 1,7-Bis((butylamino)methyl)-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine-2,8-diol
[0073] Appearance: white solid
[0074] Melting point: 159.3-161.9°C
[0075] Proton NMR spectrum: 1 H NMR (400MHz, CDCl 3 )δ6.95(d, J=8.8Hz, 2H), 6.68(d, J=8.8Hz, 2H), 4.74(d, J=2.4Hz, 4H), 4.42(d, J=16.8Hz, 2H) ,4.22(s,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com