Halohydrin dehalogenase mutant and applications thereof
A technology of halohydrin dehalogenase and mutants, which is applied in the field of molecular biology and biology, and can solve the problems of low enzyme activity, few types of halohydrin dehalogenase, and low product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Construction of wild-type halohydrin dehalogenase recombinant bacteria
[0050] Primers were designed according to the gene sequence of halohydrin dehalogenase, with the upstream primer F_HheA AM -CGC GGATCC ATGCGTATCGCTCTGGTGACG (underlined base is the recognition site of restriction endonuclease BamH I) and downstream primer R_HheA AM -CCC AAGCTT TTACGGCAGATAGCCGCCAG (underlined base is the recognition site of restriction endonuclease Hind III) to plasmid pUC57-HheA AM (synthesized by Sangon Bioengineering (Shanghai) Co., Ltd., optimized for expression codons in E.coli) for PCR amplification to obtain the target gene.
[0051] Wherein, the reaction system of PCR is:
[0052]
[0053] The PCR program is: 98°C for 2min; 98°C for 10s, 58°C for 15s, 72°C for 10s (generally 5s / kb), 30 cycles; 72°C for 5min; 4°C for heat preservation.
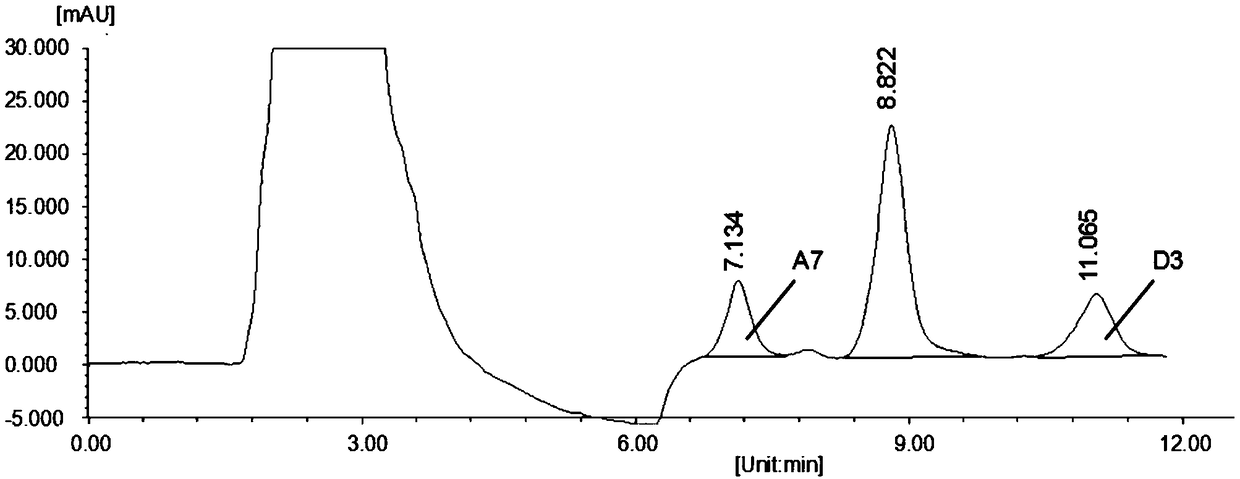
[0054] After the size of the PCR product was verified by 1% agarose gel electrophoresis, it was purified and recovered with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com