Method for preparing cyclopropanecarboxylic acid by using heteropolyacid catalyst
A technology for cyclopropanecarboxylic acid and isopropyl cyclopropanecarboxylate, which is applied in the field of preparation of cyclopropanecarboxylic acid, can solve the problems that equipment is easy to cause corrosion, is difficult to separate, and has low catalyst reuse rate, and achieves easy separation, low corrosiveness, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
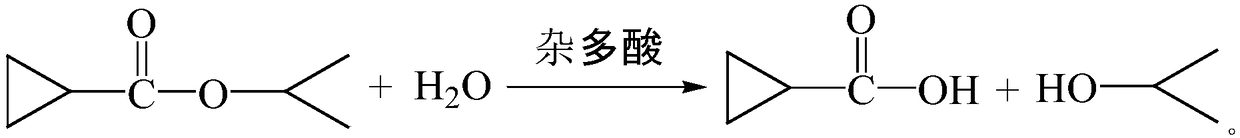
[0017] 192g cyclopropanic acid isopropionic acid was put into the flask, added 54g water, and stirred evenly. Add 5g of silicotungstic acid, stir well and react at room temperature for 2h. After the reaction was completed, filter, collect the silicotungstic acid catalyst for mechanical use, and carry out atmospheric distillation of the mother liquor, and collect 118.3 g of fractions at 80-82°C in total, with a conversion rate of 91.71%. As detected by GC, the content of cyclopropanecarboxylic acid was 99.63%.
Embodiment 2
[0019] 192g of isopropyl cyclopropanate was dropped into the flask, 54g of water was added, and stirred evenly. Add 4.3g of silicotungstic acid, stir well and react at room temperature for 2h. After the reaction was completed, filter, collect the phosphotungstic acid catalyst for mechanical use, and carry out atmospheric distillation of the mother liquor, and collect 116.8 g of 80-82 ° C fractions in total, with a conversion rate of 90.54%. As detected by GC, the content of cyclopropanecarboxylic acid is 99.59%.
Embodiment 3
[0021] 192g of isopropyl cyclopropanate was dropped into the flask, 54g of water was added, and stirred evenly. Add 5g of silicotungstic acid collected in Example 1, stir well and react at room temperature for 2h. After the reaction was completed, filter, collect the silicotungstic acid catalyst and continue to use it mechanically. The mother liquor was distilled at atmospheric pressure, and a total of 117.6g of 80-82°C fractions were collected, with a conversion rate of 91.16%. As detected by GC, the content of cyclopropanecarboxylic acid was 99.61%. After applying mechanically 10 times of reaction, the conversion rate is still 90%, and the content of cyclopropanecarboxylic acid collected is more than or equal to 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com