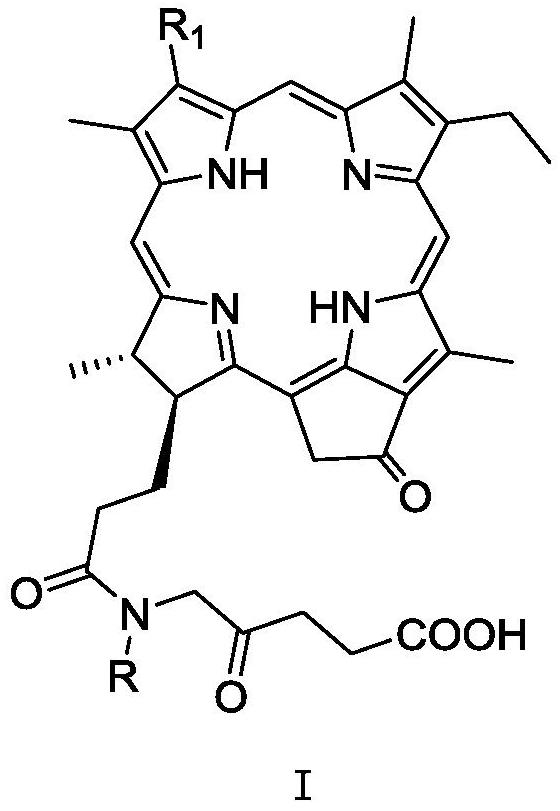
A Novel Pyropheophorbide A Derivative and Its Preparation Method and Application
A technology of pyropheophorbide and derivatives, which is applied in the field of novel pyropheophorbide a derivatives and their preparation, and can solve the problems of fast metabolism of talaporfin, easy oxidation of moporfin and poor stability. and other problems, to achieve the effect of reducing skin phototoxicity, easy preparation and strong photodynamic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
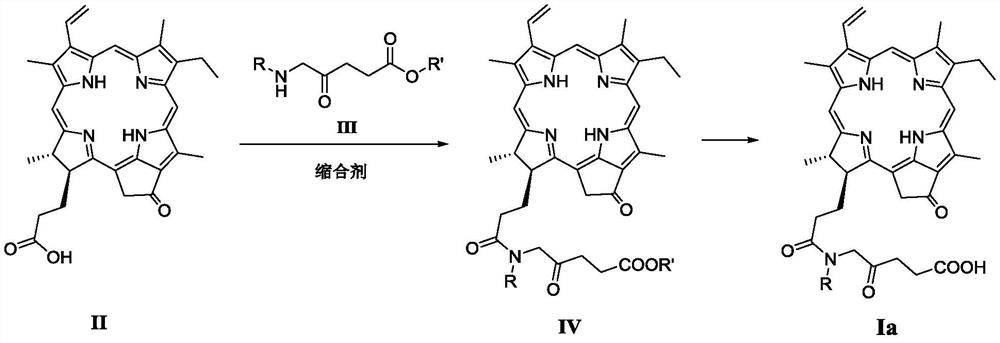
[0073] 17 3 -The preparation method of [N-(2-oxo-4-carboxybutyl)] pyropheophytin (compound Ia-1) specifically comprises the following steps:
[0074]
[0075] Step 1): 17 3 -Synthesis of [N-(2-oxo-4-methoxycarbonylbutyl)]pyropheophytin
[0076] In a 50mL round bottom flask, pyropheophorbide a (2.0g, 3.75mmol), methyl 5-aminolevulinate hydrochloride (1.0g, 5.5mmol), HBTU (1.7g, 4.49mmol ) was dissolved in dichloromethane (100mL), then DIPEA (2.0mL) was added, and stirred at room temperature for 6h. Wash three times with water (100mL×3), combine the organic phase, anhydrous MgSO 4 Dry, filter, concentrate under reduced pressure to remove the solvent, and purify by silica gel column chromatography, the eluent is dichloromethane:acetone (v / v=50:1), to obtain 17 3 -[N-(2-oxo-4-methoxycarbonylbutyl)]pyropheophytin (2.35g), yield 94.9%. 1 H NMR (400MHz, CDCl 3 )δppm: 9.48(s,1H),9.40(s,1H),8.62(s,1H),8.01(dd,J=18.2,11.7Hz,1H),6.31(d,J=17.8Hz,1H), 6.20(d, J=11.6Hz, 1H), 5.61(...
Embodiment 2
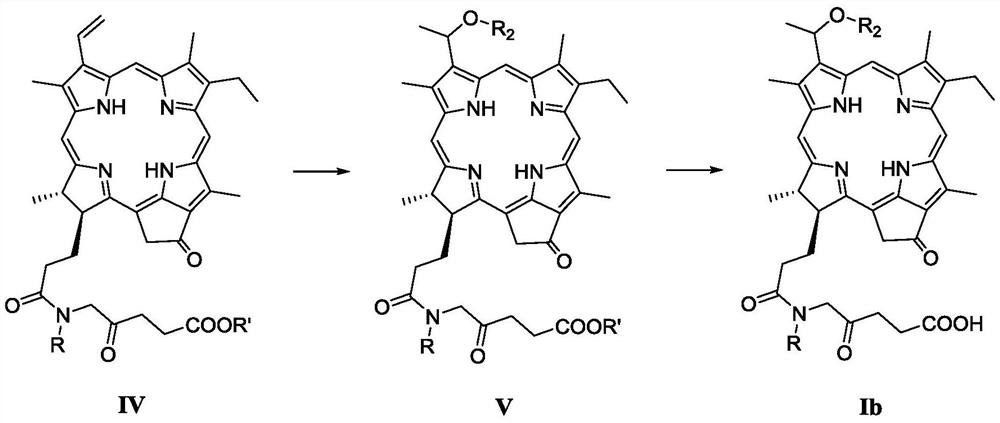
[0080] 3-(1-Methoxyethyl)-3-desvinyl-17 3 The preparation method of -[N-(2-oxo-4-carboxybutyl)] pyropheophytin (compound Ib-1) specifically comprises the following steps:
[0081]
[0082] Step 1): 3-(1-Methoxyethyl)-3-desvinyl-17 3 -Synthesis of [N-(2-oxo-4-methoxycarbonylbutyl)]pyropheophytin
[0083] to 17 3 -[N-(2-Oxo-4-methoxycarbonylbutyl)]pyropheophytin (132 mg, 0.2 mmol) was added with 5 mL of 30% HBr-AcOH solution, and stirred for 2 h. Slowly add 50mL of methanol and stir at room temperature for 4h. 100 mL of dichloromethane was added to the reaction solution, and the organic phase was washed with water three times. The organic phase was collected and washed with anhydrous MgSO 4 Dry, filter, collect the filtrate, remove the solvent under reduced pressure, and purify by silica gel column chromatography, the eluent is dichloromethane:acetone (v / v=50:1), to obtain 3-(1-methoxyethyl)- 3-Devinyl-17 3 -[N-(2-Oxo-4-methoxycarbonylbutyl)]pyropheophytin (91 mg), yie...
Embodiment 3
[0087] 17 3 - Preparation of [N-methyl-N-(2-oxo-4-carboxybutyl)] pyropheophytin (compound Ia-2)
[0088]
[0089] Using pyropheophytin-a and methyl 5-methylamino-4-oxopentanoate as raw materials, the method obtained with reference to Example 1:
[0090] 17 3 -[N-Methyl-N-(2-oxo-4-carboxybutyl)]pyropheophytin. 1 H NMR (400MHz, DMSO-d 6): δ ppm 12.33(s,1H),9.70(d,J=3.9Hz,1H),9.62(d,J=1.4Hz,1H),8.84(s,1H),5.98(dt,J=6.8, 3.9Hz, 1H), 5.19(s, 1H), 5.13(s, 1H), 4.57(dd, J=16.3, 7.9Hz, 1H), 4.33-4.21(m, 1H), 3.66(q, J=7.6 Hz, 3H), 3.54(s, 4H), 3.49(d, J=1.8Hz, 3H), 3.44(s, 2H), 3.21(s, 3H), 2.92-2.73(m, 8H), 2.69(s ,4H),2.47-2.39(m,6H),2.18(s,1H),2.04(dd,J=6.6,2.2Hz,3H),1.76(d,J=7.3Hz,3H),1.60(t, J=7.5Hz, 3H), 1.36(s, 4H), 0.19(s, 1H), -1.99(d, J=2.4Hz, 1H); ESI-MS m / z: 662.4[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com