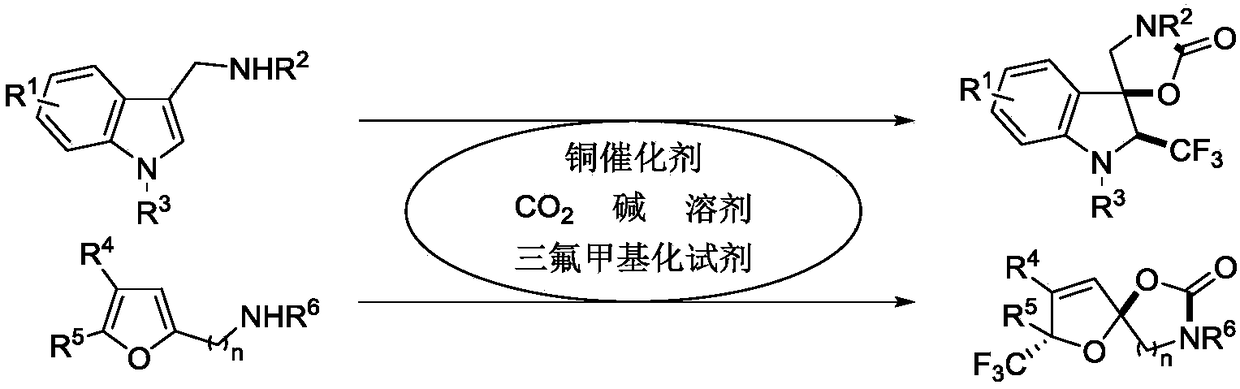
Synthetic method of trifluoromethyl-containing spiro indoline or acetal
A technology of trifluoromethylspirocyclic indoline and trifluoromethylspirocyclic acetal, which is applied in the field of compound synthesis and achieves the effects of high chemistry, good functional group compatibility, and good economic and environmental significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
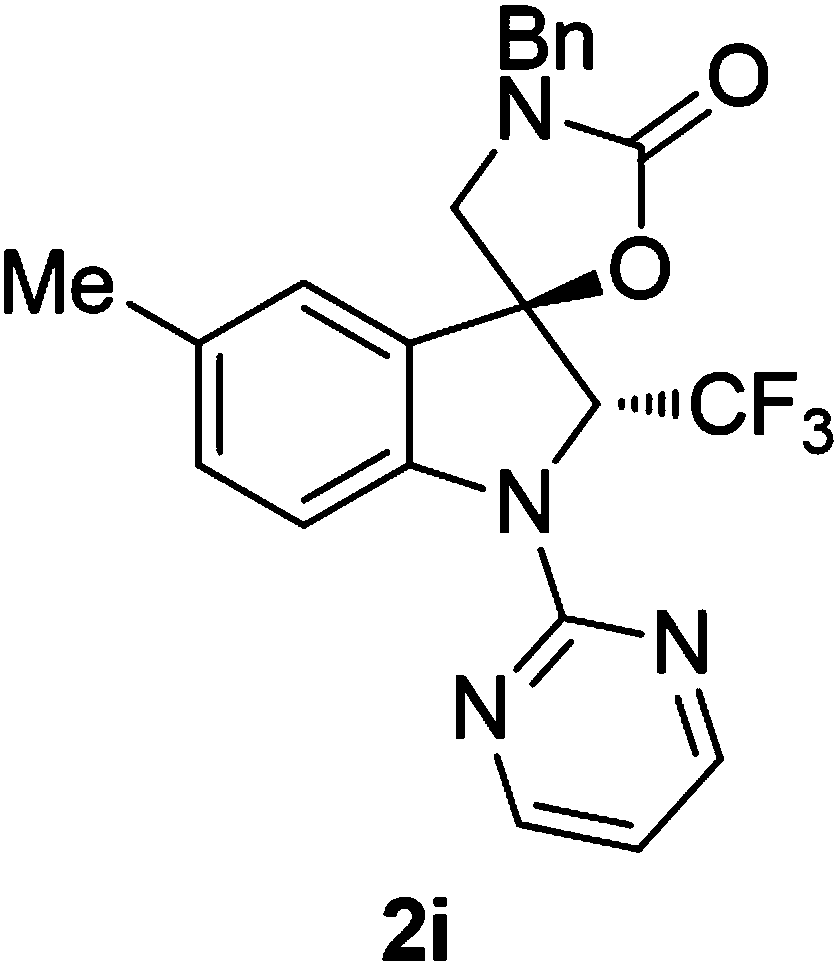
[0070] 3'-Benzyl-1-(pyrimidin-2-yl)-2-(trifluoromethyl)spiro[indoline-3,5'-oxazolidinin]-2'-one
[0071] Preparation of 3'-benzyl-1-(pyrimidin-2-yl)-2-(trifluoromethyl)spiro[indoline-3,5'-oxazolidin]-2'-one (hereinafter referred to as compound 2a) (Description: indole Line compound 2a means that the compound is prepared from substrate 1a, and the names of other indoline compounds are consistent with it)
[0072]
[0073] Optimum selection of reaction conditions in table 1 [a]
[0074]
[0075]
[0076] [a] Reaction conditions: 1a (0.2mmol), 1 atmosphere of CO 2 , second-generation Togni reagent (0.4mmol), base (0.4mmol), copper catalyst (0.02mmol), solvent (2mL), room temperature (rt), 16 hours. [b] Yields are isolated yields. [c] Second generation Togni reagent (0.3 mmol). [d] Second generation Togni reagent (0.35 mmol). Pym = 2-pyrimidinyl. TMG = tetramethylguanidine. DABCO = 1,4-diazabicyclo[2.2.2]octane. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene. DBN = 1,5-...
Embodiment 1-2 to Embodiment 1-19
[0082] Preparation of Example 1-2 to Example 1-19 Indoline Compound 2b-2s
[0083] The specific operation is as follows: the synthesis steps are consistent with the synthesis steps of compound 2a.
[0084] The preparation of table 2 indoline compounds [a]
[0085]
[0086]
[0087] [a] Reaction condition 1 (0.2mmol), 1 atmosphere CO 2 , second generation Togni reagent (0.35mmol), DBN (0.4mmol), tetraethylcyanide copper hexafluorophosphate (0.02mmol), MeCN (2mL), room temperature, 16 hours; isolated yield; all diastereomers Body selectivity (dr value) greater than 19:1, except product 2n (dr=11:1, 19 The results obtained by F-NMR analysis). [b] DMSO was used as solvent, the second generation Togni reagent (0.40mmol), 24 hours.
[0088] 3'-Butyl-1-(pyrimidin-2-yl)-2-(trifluoromethyl)spiro[indoline-3,5'-oxazolidinin]-2'-one (2b)
[0089] 3'-butyl-1-(pyrimidin-2-yl)-2-(trifluoromethyl)spiro[indoline-3,5'-oxazolidin]-2'-one
[0090] 2H),3.54–3.32(m,2H),1.73–1.64(m,2H)...
Embodiment 3
[0198] The gram-scale synthesis of embodiment 3 product 4a
[0199] The specific operation is: in the glove box, place the weighed tetraethylcyanide copper hexafluorophosphate (149mg, 0.40mmol, 0.10equiv) and the second generation Togni reagent (2.53g, 8mmol, 2.0equiv) in sequence in advance The baked 25ml Shrek tube equipped with a stirrer, then tighten the reaction tube and take it out of the glove box, and replace it with carbon dioxide gas for three times, and then under the carbon dioxide gas flow, add the substituted furan substrate 3a ( 766mg, 5.0mmol, 1.0equiv), anhydrous acetonitrile (40mL), DBU (1.20mL, 8.0mmol, 2.0equiv). After the addition operation was completed, the reaction tube was tightened in an atmosphere of carbon dioxide at atmospheric pressure, and then it was placed on a stirrer at room temperature and stirred for 16 hours. After the reaction, the reaction solution was removed from the reaction solvent on a rotary evaporator, and then the residue was se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com