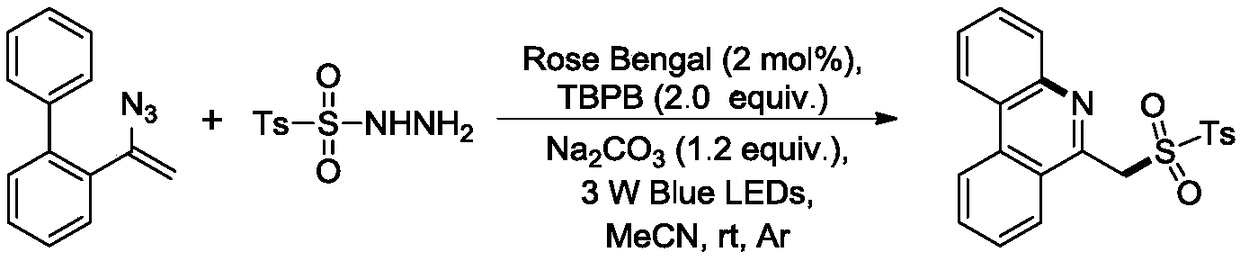
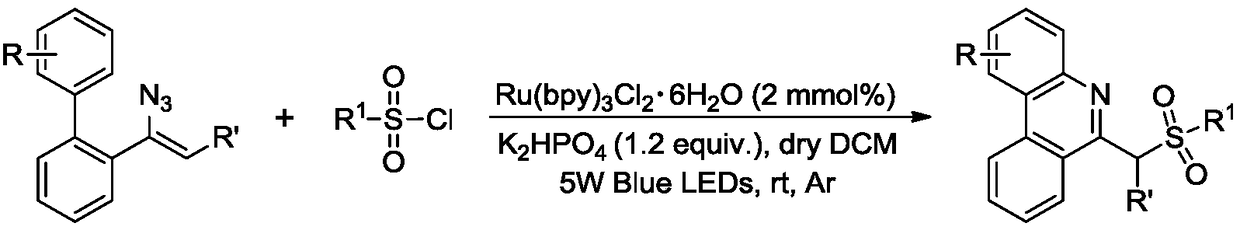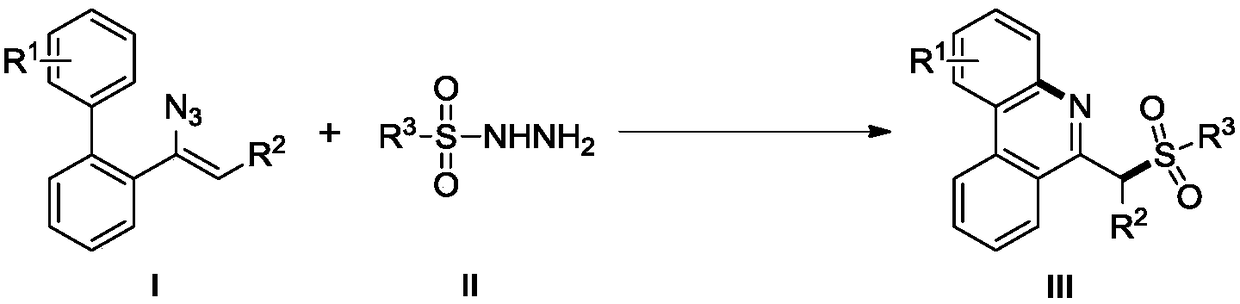Method for synthesizing 6-sulfurylmethylphenanthridine derivatives through visible light catalysis
A technology for sulfomephridine and derivatives, which is applied in the field of visible light catalyzed synthesis of 6-sulfomephridine derivatives, can solve the problems of large functional group limitations, residual metal ions, complicated operation procedures, etc., and achieves the reaction time. Short, low production cost, the effect of simplifying the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] At room temperature, add magneton, 2-(1-azidovinyl)-1,1'-biphenyl (0.26mmol, 57.5mg), p-toluenesulfonyl hydrazide (0.2mmol, 37.2mg ), Rose Bengal (0.004mmol, 4.07mg), TBPB (0.4mmol, 77.6mg) and Na 2 CO 3 (0.24mmol, 25.44mg), after changing the argon three times under vacuum, add 2ml of acetonitrile, react under 3W blue light irradiation for 10h, after the reaction is over, evaporate the solvent to obtain the crude product, then separate and purify by column chromatography to obtain the present embodiment 6- The product of sulfonemethylphenanthidine was 63.1 mg, the yield was 91%, and it was a pale yellow solid. 1 H NMR (400MHz, CDCl 3 )δ8.64(d,J=8.3Hz,1H), 8.60–8.50(m,1H),8.36(d,J=8.2Hz,1H),7.84(ddd,J=9.8,6.0,4.9Hz,2H ),7.74(t,J=7.6Hz,1H),7.67(dd,J=6.2,3.4Hz,2H),7.56(d,J=8.2Hz,2H),7.18(d,J=8.0Hz,2H ),5.15(s, 2H), 2.38(s,3H). 13 C NMR (101MHz, CDCl 3 )δ149.76,144.61,143.25,135.54,133.78,133.16, 133.10,130.85,129.86,129.39,128.64,127.59,127.58,126.98,125...
Embodiment 2
[0032]
[0033] At room temperature, add magneton, 2-(1-azidovinyl)-4'-methyl-1,1'-biphenyl (0.26mmol, 61.1mg), p-toluenesulfonyl hydrazide (0.2mmol, 37.2mg), Rose Bengal (0.004mmol, 4.07mg), TBPB (0.4mmol, 77.6mg) and Na 2 CO 3 (0.24mmol, 25.44mg), after changing the argon three times under vacuum, add 2ml of acetonitrile, react under 3W blue light irradiation for 10h, after the reaction is over, evaporate the solvent to obtain the crude product, then separate and purify by column chromatography to obtain the present embodiment 6- The product of sulfonemethylphenanthidine was 66.5 mg, the yield was 92%, and it was a pale yellow solid. 1 H NMR (400MHz, CDCl 3 )δ8.56(d, J=8.3Hz, 1H), 8.40(d, J=8.4Hz, 1H), 8.29(d, J=8.2Hz, 1H), 7.85–7.76(m, 1H), 7.71– 7.61(m, 2H), 7.56(d, J=8.3Hz, 2H), 7.47(dd, J=8.4, 1.5Hz, 1H), 7.17(d, J=8.0Hz, 1H), 5.12(s, 2H ), 2.53(s,3H),2.37(s,3H). 13 C NMR (101MHz, CDCl 3 )δ149.65, 144.59, 143.45, 138.83, 135.67, 133.18, 130.76, 129.43, 129.38, 1...
Embodiment 3
[0035]
[0036] At room temperature, add magneton, 2-(1-azidovinyl)-4'-methoxy-1,1'-biphenyl (0.26mmol, 65.3mg), p-toluenesulfonyl Hydrazine (0.2mmol, 37.2mg), Rose Bengal (0.004mmol, 4.07mg), TBPB (0.4mmol, 77.6mg) and Na 2 CO 3 (0.24mmol, 25.44mg), after changing the argon three times under vacuum, add 2ml of acetonitrile, react under 3W blue light irradiation for 10h, after the reaction is over, evaporate the solvent to obtain the crude product, then separate and purify by column chromatography to obtain the present embodiment 6- The product of sulfonemethylphenanthidine was 65.6 mg, the yield was 87%, and it was a pale yellow solid. 1 H NMR (400MHz, DMSO) δ8.71 (d, J = 7.6Hz, 1H), 8.65 (d, J = 8.5Hz, 1H), 8.38 (d, J = 7.5Hz, 1H), 7.88 (s, 1H ),7.64(d,J=6.9Hz, 3H),7.35(d,J=6.9Hz,3H),7.26(s,1H),5.38(s,2H),3.90(s,3H),2.37(s ,3H). 13 C NMR(101 MHz,DMSO)δ160.20,151.10,144.96,144.82,136.74,133.17,131.58,130.00,128.65,128.06, 126.87,124.80,124.43,122.41,118.75,117.95,109.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com