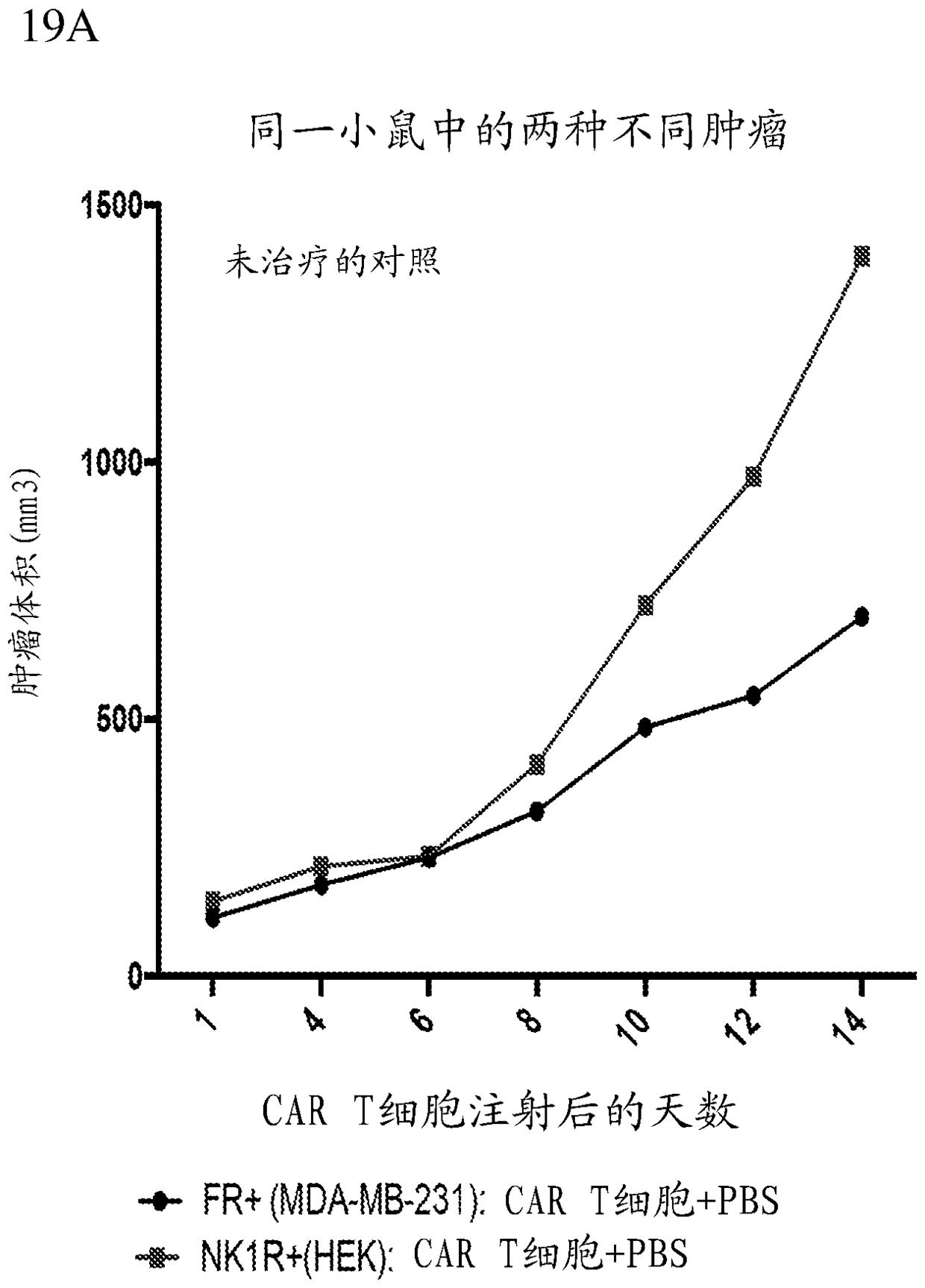
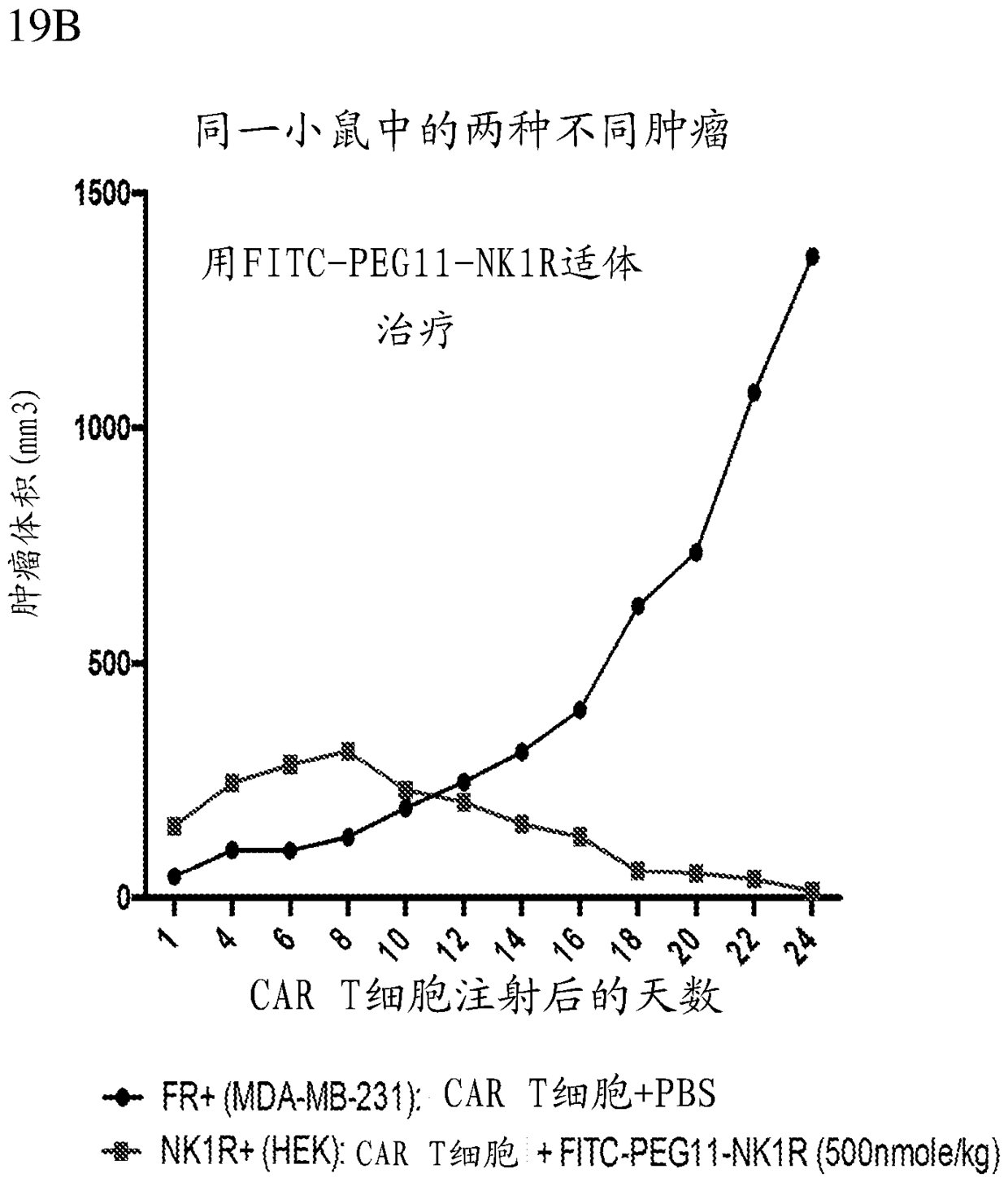
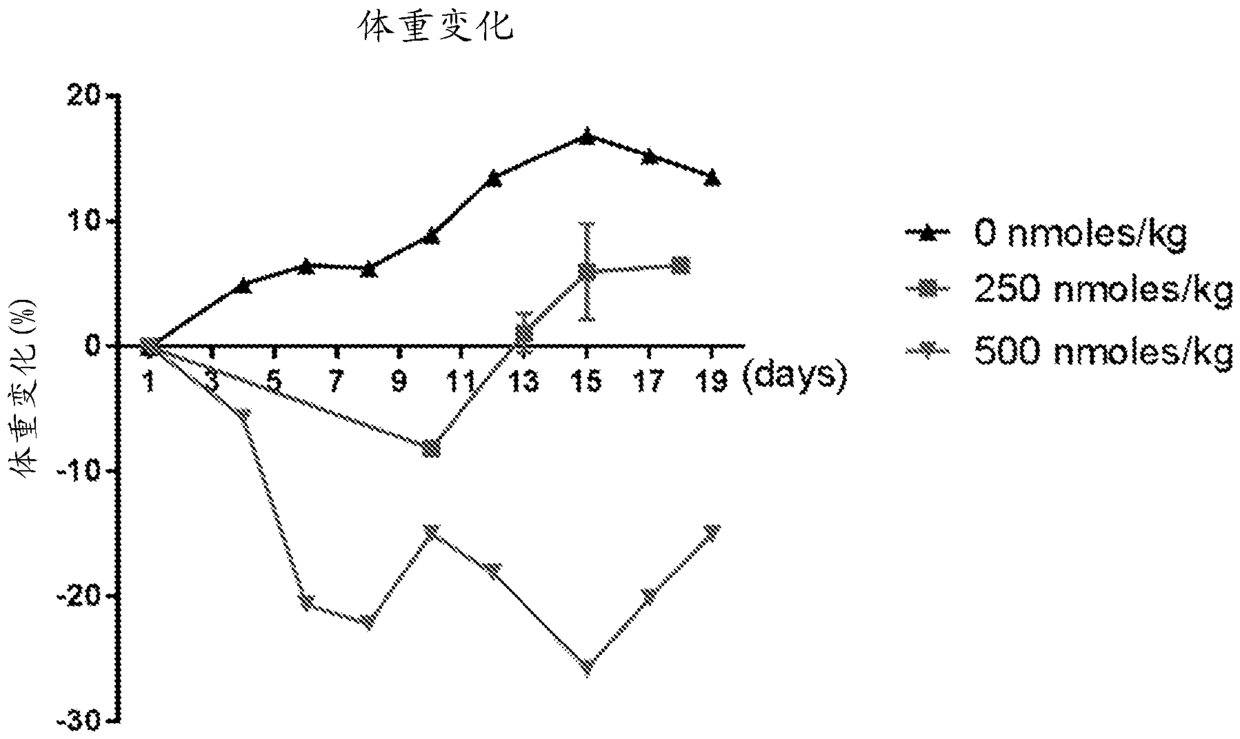
Methods and compositions for CAR T cell therapy
A composition and cell technology, applied in the field of CAR T cell therapy and composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0309] Preparation of lentiviral vector encoding CAR gene
[0310] An overlap PCR approach was used to generate CAR constructs containing scFv against fluorescein. A scFv against fluorescein was synthesized, namely 4M5.3 (Kd = 270 fM, 762 bp) derived from an anti-fluorescein (4-4-20) antibody. Encoding human CD8α signal peptide (SP, 63 bp), hinge and transmembrane region (249 bp), cytoplasmic domain of 4-1BB (CD137, 141 bp) as shown in Figure 14A by overlap PCR pairs) and the sequence of the CD3ζ chain (336 bp) were fused to anti-fluorescein scFv. The resulting CAR construct (1551 bp) was inserted into the EcoRI / NotI cut lentiviral expression vector pCDH-EF1-MCS-(PGK-GFP) ( FIG. 14B , System Biosciences). The sequence of the CAR construct in the lentiviral vector was confirmed by DNA sequencing.
[0311] An exemplary CAR nucleic acid coding sequence may comprise:
[0312]
[0313] .
[0314] In the exemplary nucleic acid sequences shown above, the first ATG is the ...
Embodiment 2
[0322] Preparation of Lentivirus Containing CAR Gene for Transduction of Human T Cells
[0323] To generate lentivirus containing an anti-fluorescein single-chain fragment variable (scFv) CAR, the 293TN packaging cell line was co-transfected with a mixture of lentiviral vector encoding anti-fluorescein scFvCAR and a second-generation packaging plasmid (Cellecta). After 24 and 48 hours of transfection, supernatants containing lentiviruses with CAR genes were harvested and viral particles were concentrated for future human T cell transductions by standard polyethylene glycol virus enrichment methods (Clontech) .
Embodiment 3
[0325] Isolation of Human T Cells from Human PBMCs
[0326] T cells were isolated from human peripheral blood mononuclear cells (PBMC) by Ficoll density gradient centrifugation (GE Healthcare Lifesciences). After washing away the residual Ficoll solution, T cells were isolated using the EasySep™ Human T Cell Isolation Kit (STEM CELL technologies). Purified T cells were cultured in TexMACS™ medium (Miltenyi Biotech Inc) containing 1% penicillin and streptomycin sulfate in the presence of human IL-2 (100 IU / mL, Miltenyi Biotech Inc). In multi-well plates at 1x10 6 T cells were cultured at a density of 1 cell / mL. T cells were split and re-fed every 2-3 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com