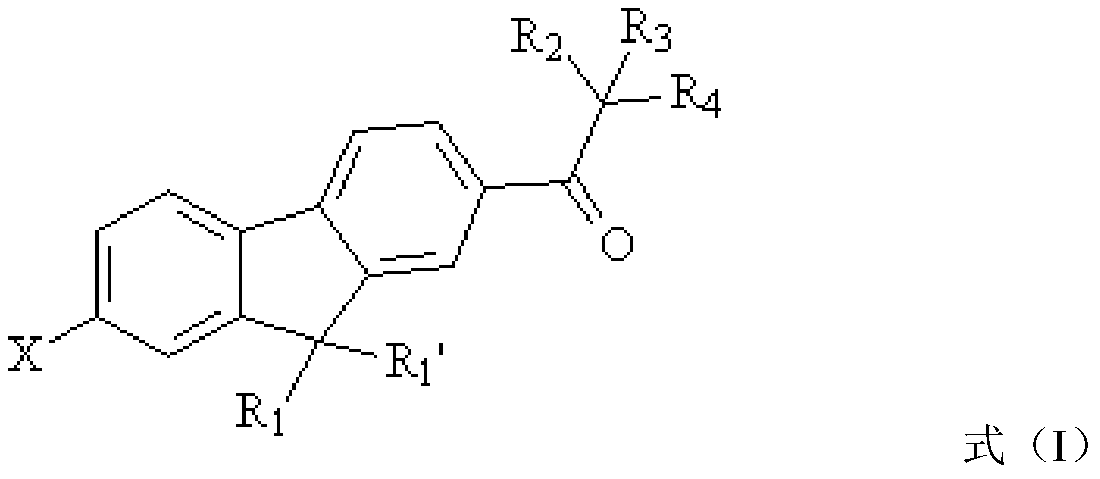
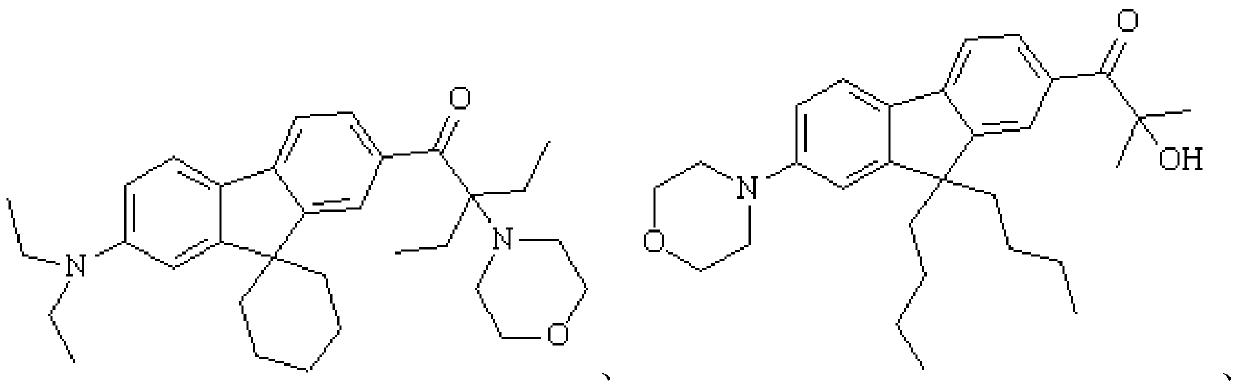
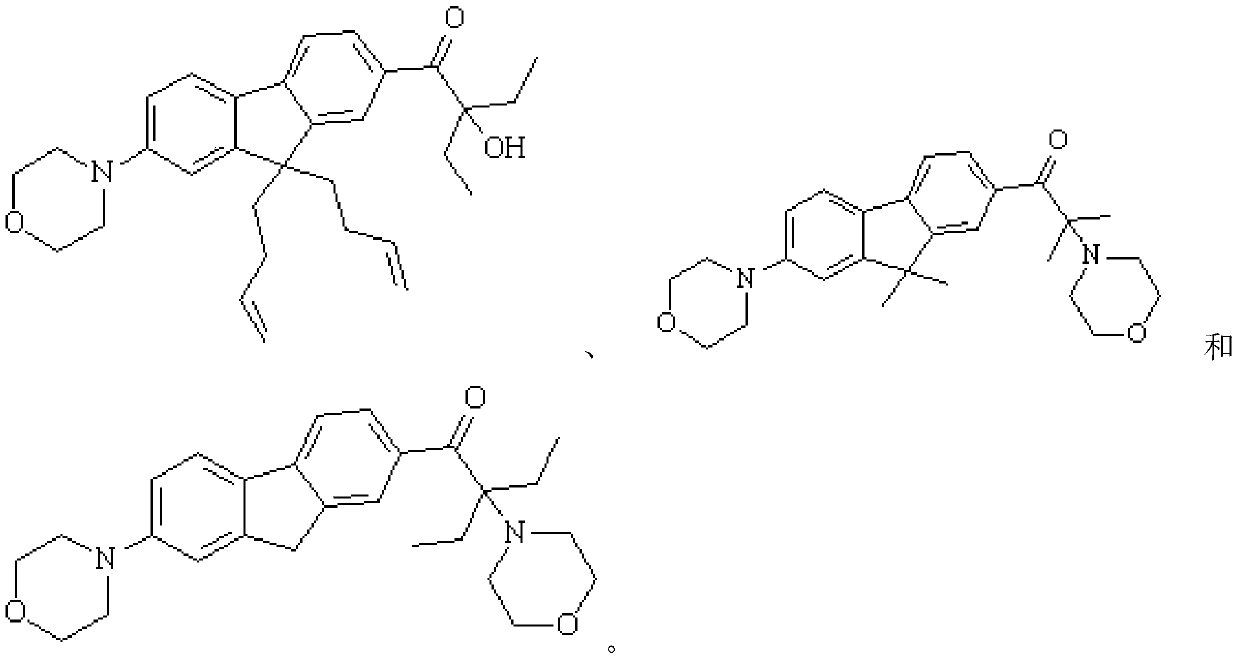
Fluorene-based polyfunctional photoinitiator, preparation method and application thereof
A photoinitiator and multi-functionality technology, which is applied in the field of photocuring, can solve the problems of low quantum absorption rate of long wavelengths, achieve high quantum absorption rate, improve initiation efficiency, and improve quantum absorption efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] Another aspect of the present application also provides a method for preparing the above-mentioned fluorene-based multifunctional photoinitiator. The preparation method includes: bromination reaction: bromination reaction of raw material a, bromination reagent and first organic solvent to obtain The intermediate product b and the raw material a have the structure shown in formula (II),
[0045]
[0046] The intermediate product b has the structure of formula (Ⅲ),
[0047]
[0048] Substitution reaction: the intermediate product b, the substitution reagent and the second organic solvent are used to undergo a substitution reaction to obtain a fluorene multifunctional photoinitiator.
[0049] According to the conventional understanding of those skilled in the art, the substituent R in the structural formula shown in formula (II) and formula (III) 1 , R 2 , R 3 , R 4 And X has the same structure as the corresponding group in formula (I). The chemical reaction process of the above...
specific Embodiment approach
[0075] The present invention will be further described in detail below in conjunction with specific embodiments, but it should not be understood as a limitation to the protection scope of the present invention.
[0076] 1. Preparation of photoinitiator.
preparation Embodiment 1
[0078] (1) Substitution reaction: preparation of intermediate 1a.
[0079] Add 36.5g of raw material 1a, 18g of N-bromosuccinimide, and 100ml of propylene carbonate to a 500mL four-necked flask, stir at room temperature, and follow the liquid phase until the raw materials no longer change, then slowly pour the materials into 1000g. In ionized water, stirring while adding, a large amount of solids precipitate out, filtered with suction, washed with water, and absolute ethanol to obtain 37.2 g of intermediate 1a. The synthetic route is as follows:
[0080]
[0081] The structural characterization data of the intermediate product 1a is as follows: 1 H-NMR(CDCl 3 , 500MHz): 0.9146 ~ 1.0002 (6H, t), 1.2878 ~ 1.3328 (8H, m), 1.4844 (6H, s), 1.8754 ~ 2.1045 (5H, m), 7.5801 ~ 8.0837 (6H, m).
[0082] MS(m / z):444(M+1) + .
[0083] (2) Substitution reaction: preparation of compound 1.
[0084] Add 22.2g of Intermediate 1a, 50mL of methanol, and 14.0g of sodium methoxide to a 500mL four-necked f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com