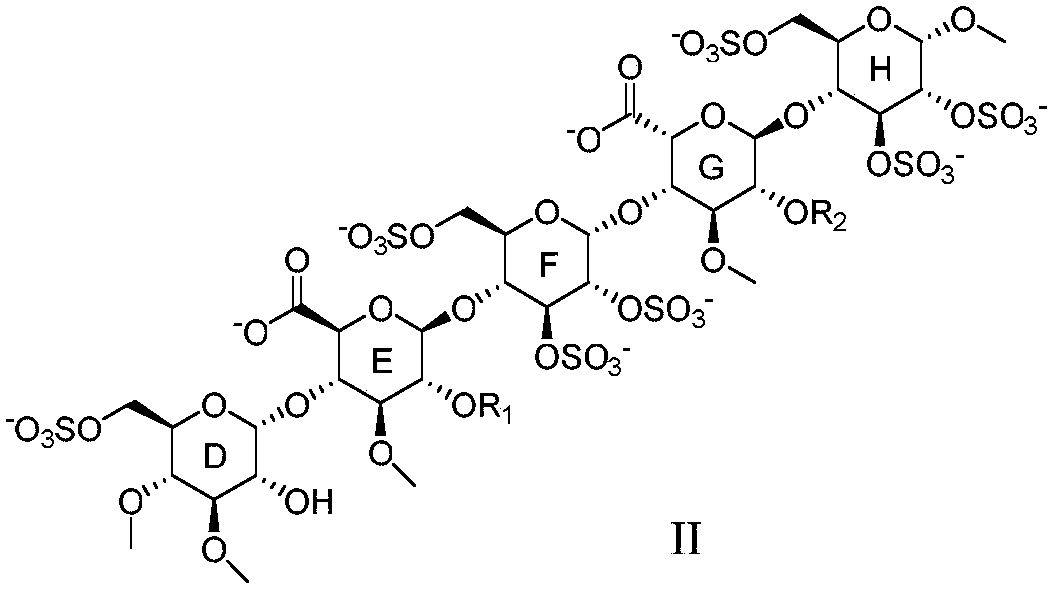
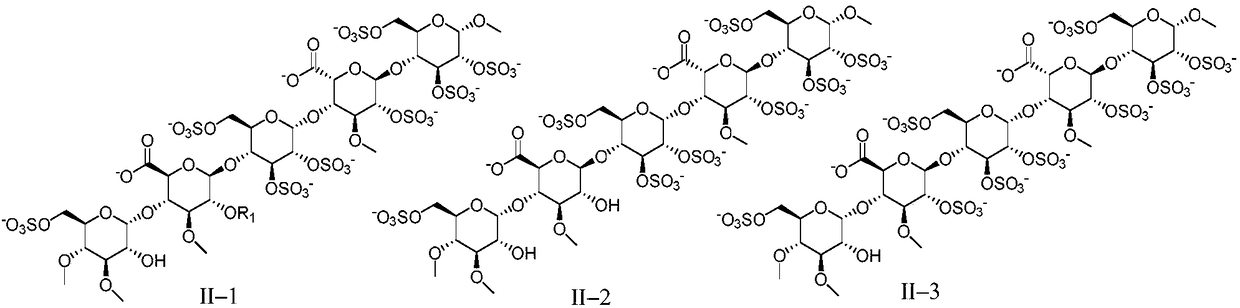
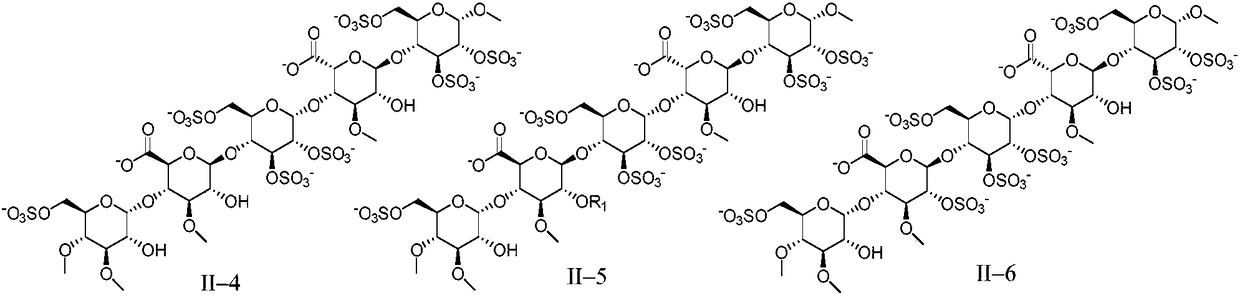
Anti-coagulation pentasaccharide compound and preparing method and medical application thereof
A technology of compound and sulfation reaction, which is applied in the direction of sugar derivatives, pharmaceutical formulas, and medical preparations containing active ingredients, etc., can solve the problems of increased bleeding risk, achieve the effects of simplified preparation methods, small dosages, and reduced raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0083] Preparation of compound D1: Dissolve D0 (748g) in tetrahydrofuran, use excess potassium hydroxide as a base, add dimethyl sulfate dropwise at 0°C for 2 hours, add potassium hydroxide aqueous solution to quench for 4 hours after the reaction, and then extract the fraction with ethyl acetate solution, spin-dried to obtain D1 (804g), yield 100%.
[0084] The preparation of compound D2: Add D1 (804g) to the solution of acetic acid: water: sulfuric acid = 2000g:400g:98g, add ethyl acetate and water after reflux reaction for 1h, and evaporate the organic phase to dryness to obtain D2 (690g). 89%.
[0085] Preparation of compound D3: Dissolve D2 (388g) in anhydrous dichloromethane, add 288g of trichloroacetonitrile and 15g of DBU to react for 1h, then spin to dry column chromatography to obtain D3 (490g) with a yield of 92%.
[0086] The second part preparation of monosaccharide E ring
[0087] synthetic route:
[0088]
[0089] a) dimethyl sulfate, KOH, tetrahydrofuran,...
Embodiment 1 5
[0159] Embodiment 1 pentasaccharide II-1 (R 1 for the preparation of methyl, sodium salt)
[0160] synthetic route:
[0161]
[0162] Preparation of compound DEFGH10: Dissolve DE16 (32g) and FGH5 (50g) in anhydrous dichloromethane, add TMSOTf 1g dropwise at 0°C, add triethylamine to quench after reacting for 1h, and obtain DEFGH10 (61g, Yield 81%). 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55(m,5H),7.34-7.29(m,40H),5.99(d,1H),5.60(m,4H),5.17(dd,J=9.0,3.4Hz,1H),4.64 –4.62(m,18H),4.23-4.19(m,3H),4.00(m,3H),3.80-3.77(m,8H),3.70(s,6H),3.61(d,3H),3.50(d ,3H),3.41-3.40(m,18H),3.36(m,3H).MS(ESI):1815.8[M+Na] +
[0163] Preparation of compound DEFGH11: Dissolve DEFGH10 (60 g) in anhydrous methanol, add 10% palladium carbon, reduce with hydrogen under normal pressure for 24 h, filter and spin dry to obtain DEFGH11 (34 g, yield 95%). 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55 (m, 5H), 5.99 (d, J = 3.6Hz, 1H), 5.60 (d, J = 8Hz, 1H), 5.40 (m, 3H), 5.17 (dd, J = 9.0, 3.4Hz, 1H), 4.77(d, 3H), 4...
Embodiment 2 5
[0166] The preparation of embodiment 2 pentasaccharide II-2 (sodium salt)
[0167] synthetic route:
[0168]
[0169] Referring to the preparation method of Example 1, DE15 and FGH5 were used as raw materials to react, and then the pentasaccharide II-2 (sodium salt) was obtained through hydrogenolysis reaction to remove benzyl group, sulfation reaction and hydrolysis reaction.
[0170] Compound DEFGH20: 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55(m,5H),7.34-7.29(m,40H),5.99(d,1H),5.97(d,J=3.4Hz,,1H),5.60(m,3H),5.17( dd,J=9.0,3.4Hz,2H),4.64–4.62(m,18H),4.23-4.19(m,4H),4.00(m,3H),3.80-3.77(m,6H),3.70(s, 6H),3.61(d,3H),3.50(d,3H),3.41-3.40(m,18H),3.36(m,2H),2.02(s,3H).MS(ESI):1843.8[M+Na ] +
[0171] Compound DEFGH21: 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55 (m, 5H), 5.99 (d, J = 3.6Hz, 2H), 5.40 (m, 3H), 5.17 (dd, J = 9.0, 3.4Hz, 2H), 4.77 (d, 3H ),4.71(d,2H),4.64–4.62(m,2H),4.23-4.19(m,4H),4.10(m,1H),3.94-3.80(m,10H),3.70(d,6H), 3.57-3.50(m,8H),3.41-3.40(m,15H),3.30(m,2H),2.02(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com