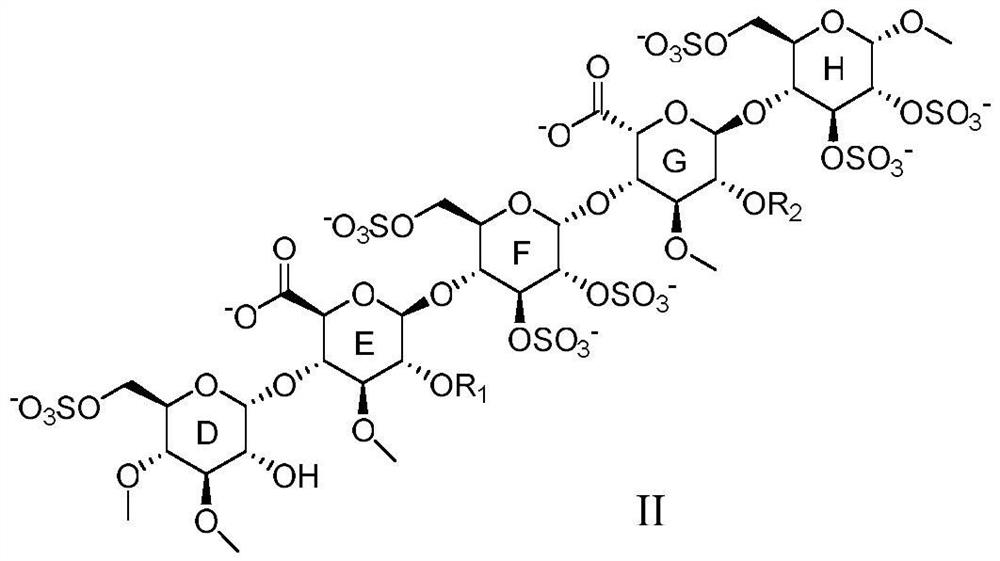
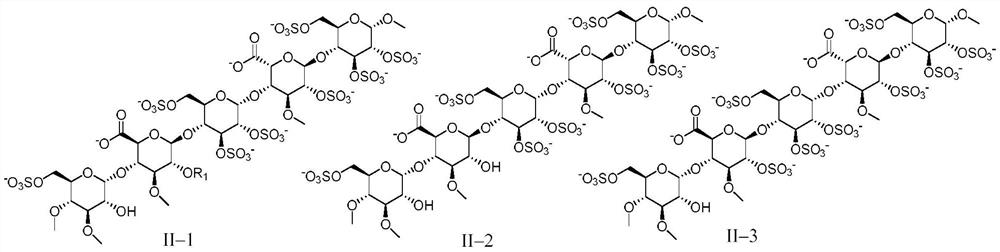
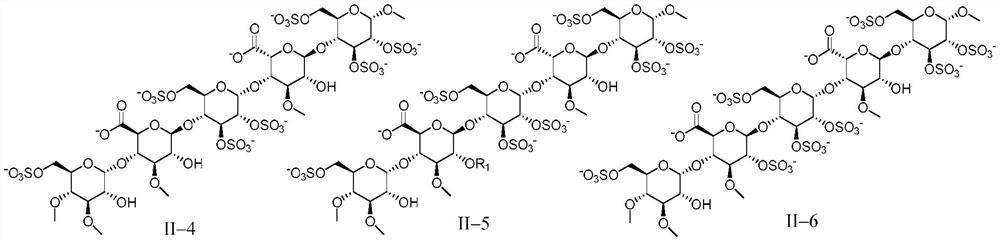
Anticoagulant pentasaccharide compound, preparation method and medical use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0083] Preparation of compound D1: Dissolve D0 (748g) in tetrahydrofuran, use excess potassium hydroxide as a base, add dimethyl sulfate dropwise at 0°C for 2 hours, add potassium hydroxide aqueous solution to quench for 4 hours after the reaction, and then extract the fraction with ethyl acetate solution, spin-dried to obtain D1 (804g), yield 100%.
[0084] The preparation of compound D2: Add D1 (804g) to the solution of acetic acid: water: sulfuric acid = 2000g:400g:98g, add ethyl acetate and water after reflux reaction for 1h, and evaporate the organic phase to dryness to obtain D2 (690g). 89%.
[0085] Preparation of compound D3: Dissolve D2 (388g) in anhydrous dichloromethane, add 288g of trichloroacetonitrile and 15g of DBU to react for 1h, then spin to dry column chromatography to obtain D3 (490g) with a yield of 92%.
[0086] The second part preparation of monosaccharide E ring
[0087] synthetic route:
[0088]
[0089] a) dimethyl sulfate, KOH, tetrahydrofuran,...
Embodiment 1 5
[0159] Embodiment 1 pentasaccharide II-1 (R 1 for the preparation of methyl, sodium salt)
[0160] synthetic route:
[0161]
[0162] Preparation of compound DEFGH10: Dissolve DE16 (32g) and FGH5 (50g) in anhydrous dichloromethane, add TMSOTf 1g dropwise at 0°C, add triethylamine to quench after reacting for 1h, and obtain DEFGH10 (61g, Yield 81%). 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55(m,5H),7.34-7.29(m,40H),5.99(d,1H),5.60(m,4H),5.17(dd,J=9.0,3.4Hz,1H),4.64 –4.62(m,18H),4.23-4.19(m,3H),4.00(m,3H),3.80-3.77(m,8H),3.70(s,6H),3.61(d,3H),3.50(d ,3H),3.41-3.40(m,18H),3.36(m,3H).MS(ESI):1815.8[M+Na] +
[0163] Preparation of compound DEFGH11: Dissolve DEFGH10 (60 g) in anhydrous methanol, add 10% palladium carbon, reduce with hydrogen under normal pressure for 24 h, filter and spin dry to obtain DEFGH11 (34 g, yield 95%). 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55 (m, 5H), 5.99 (d, J = 3.6Hz, 1H), 5.60 (d, J = 8Hz, 1H), 5.40 (m, 3H), 5.17 (dd, J = 9.0, 3.4Hz, 1H), 4.77(d, 3H), 4...
Embodiment 2 5
[0166] The preparation of embodiment 2 pentasaccharide II-2 (sodium salt)
[0167] synthetic route:
[0168]
[0169] Referring to the preparation method of Example 1, DE15 and FGH5 were used as raw materials to react, and then the pentasaccharide II-2 (sodium salt) was obtained through hydrogenolysis reaction to remove benzyl group, sulfation reaction, and hydrolysis reaction.
[0170] Compound DEFGH20: 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55(m,5H),7.34-7.29(m,40H),5.99(d,1H),5.97(d,J=3.4Hz,,1H),5.60(m,3H),5.17( dd,J=9.0,3.4Hz,2H),4.64–4.62(m,18H),4.23-4.19(m,4H),4.00(m,3H),3.80-3.77(m,6H),3.70(s, 6H),3.61(d,3H),3.50(d,3H),3.41-3.40(m,18H),3.36(m,2H),2.02(s,3H).MS(ESI):1843.8[M+Na ] +
[0171] Compound DEFGH21: 1 H NMR (300MHz, CDCl 3 )δ8.05-7.55 (m, 5H), 5.99 (d, J = 3.6Hz, 2H), 5.40 (m, 3H), 5.17 (dd, J = 9.0, 3.4Hz, 2H), 4.77 (d, 3H ),4.71(d,2H),4.64–4.62(m,2H),4.23-4.19(m,4H),4.10(m,1H),3.94-3.80(m,10H),3.70(d,6H), 3.57-3.50(m,8H),3.41-3.40(m,15H),3.30(m,2H),2.02...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com