Lithium lanthanum titanate-lithium titanate coated lithium nickel cobalt aluminate cathode material and preparation method thereof
A technology coated with nickel-cobalt lithium aluminate and lithium titanate, which is applied in positive electrodes, battery electrodes, active material electrodes, etc., can solve the problems of unsatisfactory improvement effects, achieve excellent cycle stability, accelerate migration rate, The effect of excellent electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
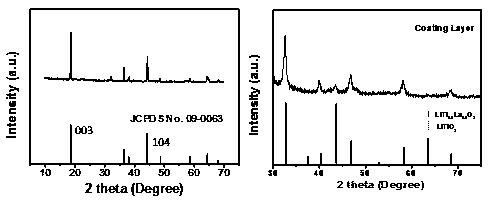
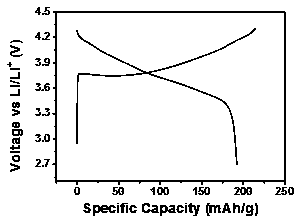
[0051] This embodiment provides a nickel-cobalt-lithium-aluminate cathode material coated with lithium lanthanum titanate-lithium titanate. Prepared by the following preparation method.
[0052] (1) Dissolve tetrabutyl titanate in an appropriate amount of ethanol solvent, after fully stirring, add a certain amount of lanthanum nitrate to make Ti:La 1:1, and then fully stir until a colorless transparent solution is formed;
[0053] (2) LiNi 0.815 co 0.15 Al 0.035 o 2 and 1wt% (relative to nickel-cobalt-lithium aluminate) PVP (polyvinylpyrrolidone) was added to the above solution, and placed in a constant water bath at 80°C to fully stir to form a uniform suspension, wherein the molar ratio of titanium to nickel-cobalt-lithium aluminate 1:100;
[0054] (3) Deionized water was added dropwise under stirring conditions to slowly hydrolyze tetrabutyl titanate, wherein the molar ratio of the amount of deionized water to the added titanium source was 2:1. Stir well until the sol...
Embodiment 2
[0059] This embodiment provides a nickel-cobalt-lithium-aluminate cathode material coated with lithium lanthanum titanate-lithium titanate. Prepared by the following preparation method.
[0060] (1) Dissolve tetraisopropyl titanate in an appropriate amount of ethylene glycol solvent, and after fully stirring, add a certain amount of lanthanum acetate to make the Ti:La ratio 1:2, and then fully stir until a colorless and transparent solution is formed;
[0061] (2) LiNi 0.9 co 0.07 Al 0.03 o 2 and 0.5wt% (relative to lithium nickel cobalt aluminate) of CTAB (cetyltrimethylammonium bromide) were added to the above solution, and placed in a constant water bath at 80 ° C to fully stir to form a uniform suspension, in which titanium and The molar ratio of nickel cobalt lithium aluminate is 0.5:100;
[0062] (3) Deionized water was added dropwise under stirring conditions to slowly hydrolyze tetrabutyl titanate, wherein the molar ratio of the amount of deionized water to the ad...
Embodiment 3
[0065] This embodiment provides a nickel-cobalt-lithium-aluminate cathode material coated with lithium lanthanum titanate-lithium titanate. Prepared by the following preparation method.
[0066] (1) Dissolve tetrabutyl titanate in an appropriate amount of glycerol solvent. After fully stirring, add a certain amount of lanthanum nitrate to make the Ti:La ratio 2:1, and then fully stir until a colorless and transparent solution is formed;
[0067] (2) LiNi 0.8 co 0.15 Al 0.05 o 2 and 2wt% (relative to nickel-cobalt-lithium aluminate) PVP (polyvinylpyrrolidone) were added to the above solution, and placed in a constant water bath at 80 ° C to form a uniform suspension, wherein the molar ratio of titanium to nickel-cobalt-lithium aluminate is 2:100;
[0068] (3) Deionized water was added dropwise under stirring conditions to slowly hydrolyze tetrabutyl titanate, wherein the molar ratio of the amount of deionized water to the added titanium source was 2:1. Stir well until the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com