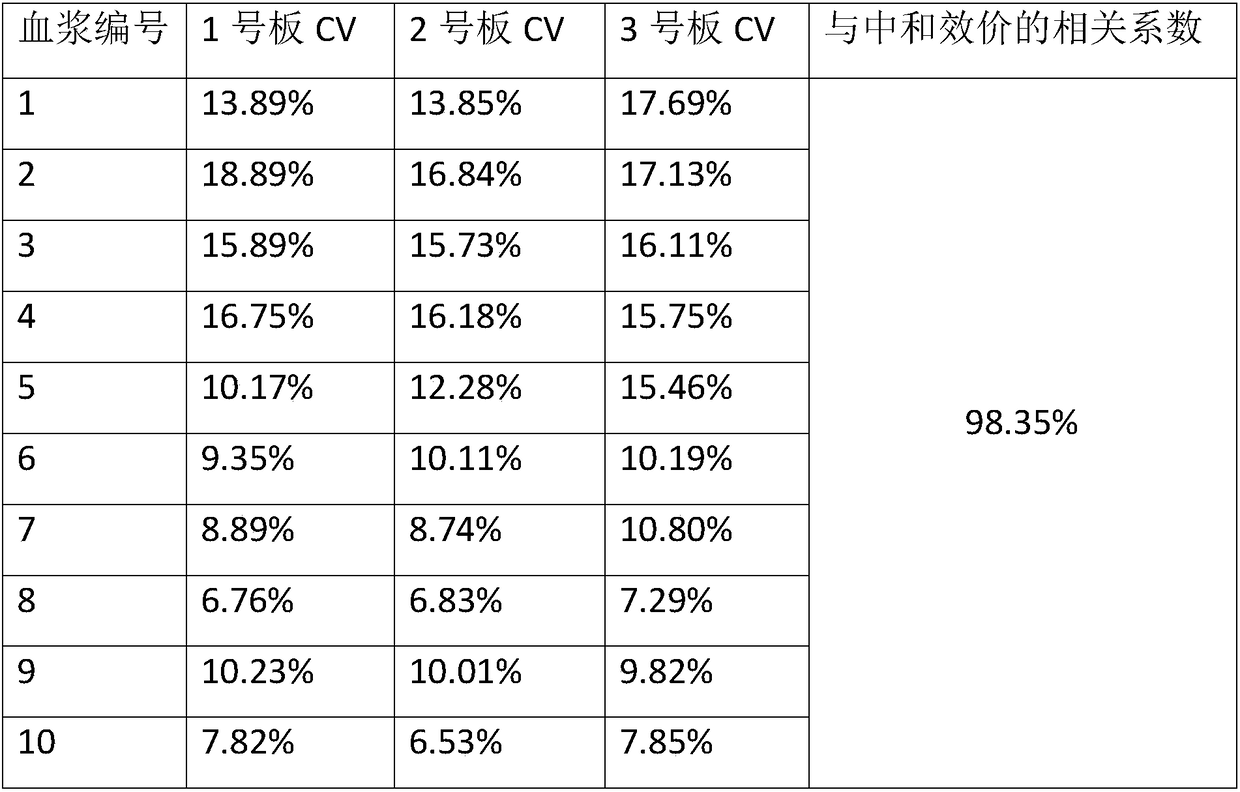
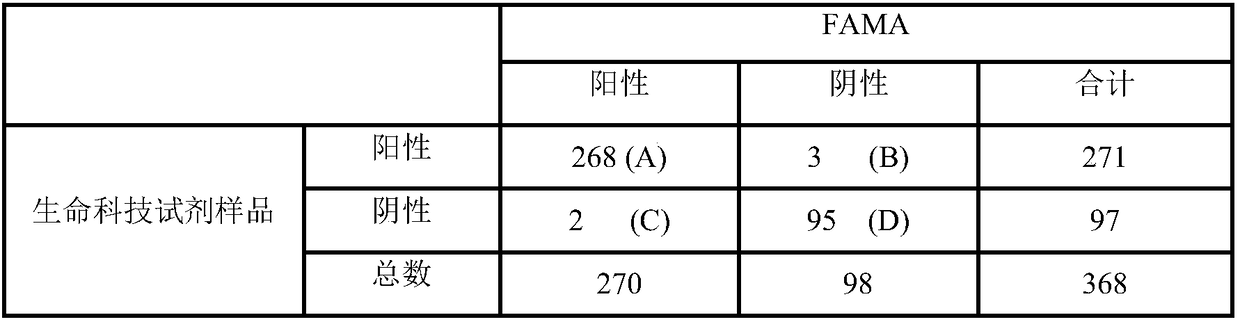
Detection kit for varicella-herpes zoster virus neutralizing antibody and detection method for varicella-herpes zoster virus neutralizing antibody
A technology of herpes zoster virus and detection kit, which can be applied to measurement devices, instruments, scientific instruments, etc., can solve problems such as poor sensitivity, and achieve the effects of accurate selection, saving production costs, and eliminating non-specific binding.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of recombinant expression of varicella-zoster virus glycoprotein E antigen
[0041] 1. Target gene amplification
[0042] (1) Primer design
[0043]According to the varicella-zoster virus glycoprotein E gene sequence published on Genbank, select the nucleic acid sequence corresponding to the amino acid sequence 25-537 to design primers with Nhe I and EcoR I restriction sites (marked in italics) at both ends:
[0044] SEQID NO.1: The forward primer is (5'-3'): tactaactagcatgaccaatccagttcgaaccagcgtac;
[0045] SEQID NO.2: The reverse primer is (5'-3'):
[0046] ttccggaattcttaatgatgatgagacagatgacgcagcagagaccttgtaaccggat;
[0047] (2) PCR amplification
[0048] The varicella-zoster virus glycoprotein E gene was amplified by RT-PCR, and the varicella-zoster virus glycoprotein E gene RT-PCR product and pET-32a(+) vector were simultaneously amplified with EcoR I and HindⅢ Enzyme digestion, recovery of the target fragment, quantification of the targe...
Embodiment 2
[0060] Example 2: Preparation of a detection kit for detecting varicella-zoster virus neutralizing antibody
[0061] The preparation of the ELISA plate is as follows: Dilute the recombinant expression varicella-zoster virus glycoprotein E antigen prepared in Example 1 to 20pg / mL, add 20ul / well to the wells of the 96-well plate, and add 5 to 10 Microliter 2% to 5% glutaraldehyde solution, shake the 96-well plate horizontally, mix well, react at room temperature for 10 minutes, and the solution in the well is solidified, ready for use; block with 0.5% bovine serum albumin at 30°C for 2 hours, wash with washing solution , pat dry.
Embodiment 3
[0062] Embodiment 3: sample detection
[0063] 1. Dilute the sample to be tested to an appropriate concentration with the sample diluent (0.5mol / L carbonate buffer solution with pH=9.5, adding bovine serum albumin with a mass concentration of 1%), and dilute the sample with the sample diluent at the same time. The human anti-VZV serum of the standard sample is serially diluted to make a standard curve; the standard sample comes from a cured varicella patient, and the titer of the human anti-VZV serum calibrated by the VZVIgG international standard.
[0064] Human anti-VZV serum (from patients with cured varicella whose potency has been calibrated by VZV IgG international standard), was diluted with the above sample diluent to 2IU / ml, 1.8IU / ml, 1.6IU / ml, 1.4IU / ml, 1.2IU / ml, 1.0IU / ml, 0.8IU / ml, 0.6IU / ml, 0.4IU / ml, 0.2IU / ml, a total of 10 concentrations. Each concentration was then diluted 100 times with R1 for use. To the wells of each cell density, add 50 microliters of human...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com