Compositions and methods for lightening skin and reducing hyperpigmentation
A technology of composition and compound, applied in the field of skin brightening, pigmentation removal, and skin care, which can solve the problem of less measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
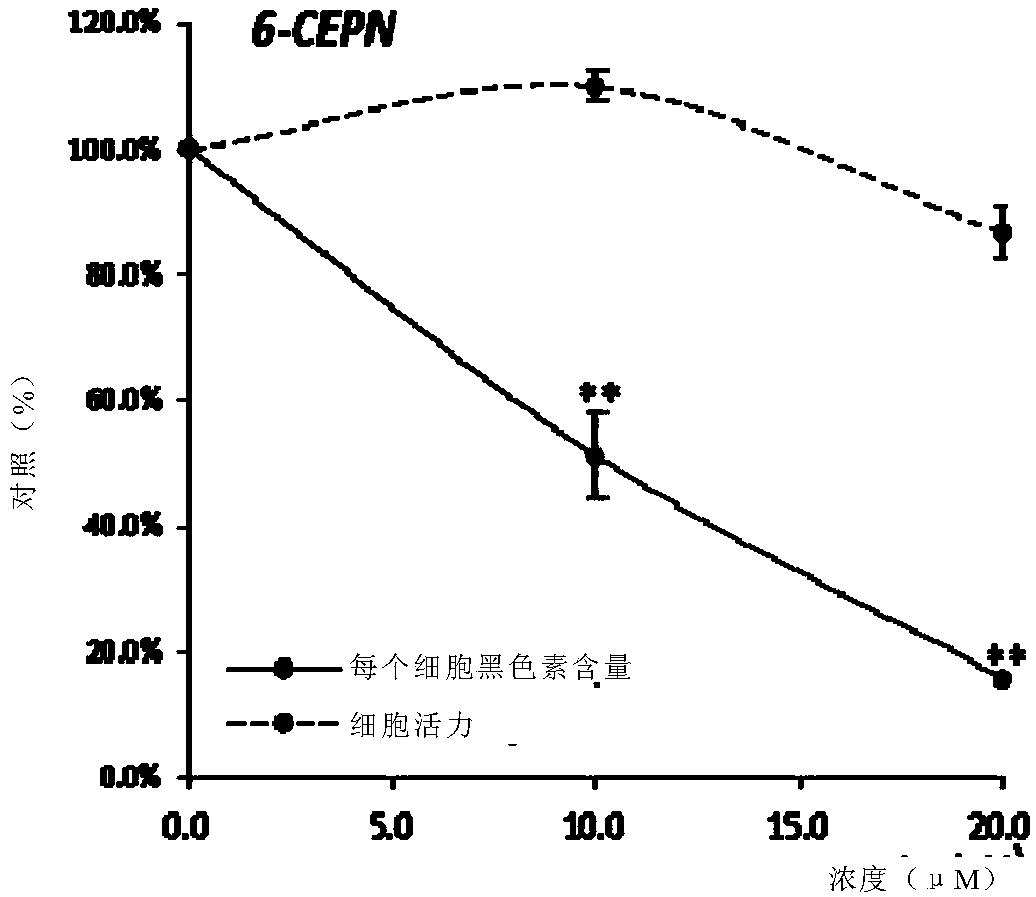
[0209] Example 1: No apparent cytotoxicity associated with 6-CEPN and significant efficacy in reducing melanin synthesis
[0210] Materials and methods
[0211] Cell Culture and Handling
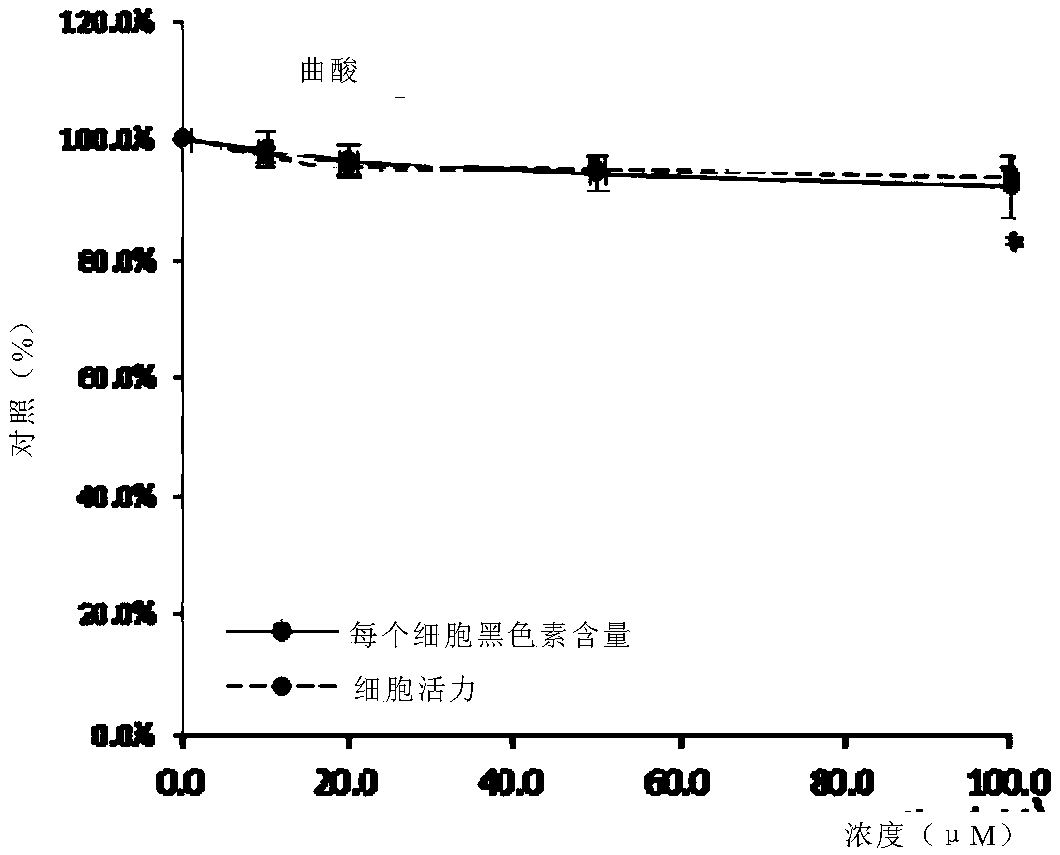
[0212] Mouse melanin A (Melan-a) cells (immortalized, non-tumorigenic mouse melanocyte cell line) were obtained from the Chinese University of Hong Kong. Cells were cultured in RPMI 1640 medium. The medium was supplemented with 10% FBS and 1% penicillin-streptomycin, and the cells were incubated at 37°C, 5% CO 2 incubate. Melanin A cells were incubated with tetradecanoylphorbol acetate (TPA) at a concentration of 200 nM. The tested chemicals (eg, 6-CEPN and kojic acid) were dissolved in 100% dimethyl sulfoxide (DMSO) at a concentration of 100 mM as stock solutions, respectively. Dilute the stock solution to the desired final concentration using culture medium immediately before use. The final DMSO concentration does not exceed 0.1%.
[0213] Assessing cell viability
[0214] Melani...
Embodiment 2
[0220] Example 2: Inhibition of tyrosinase and tyrosinase-related proteins by 6-CEPN
[0221] Materials and methods
[0222] Tyrosinase activity assay
[0223] Cellular tyrosinase activity was determined by measuring the oxidation of dopachrome in cell lysates by L-3,4-dihydroxyphenylalanine (L-DOPA). Melanin A (Melan-a) cells (1.5×10 5cells / well) were seeded in 1 mL of medium in a 24-well multiwell plate for at least 24 hours. Cells were treated with α-MSH (1 μM) alone or with α-MSH plus various concentrations of test reagents for 72 hours. Cells were washed with cold PBS and lysed in 900 μL PBS containing 1% Triton X-100. After freeze-thawing at -80°C for 30 minutes and then keeping at 25°C for 30 minutes, 100 μL of 10 mM L-DOPA was then added to each well. After incubation at 37°C for 1 hour, the absorbance of the reagents at 490 nm was measured using a Victor X4 Multi label Plate Reader (PerkinElmer, MA, USA) .
[0224] western blot
[0225] Melanin A (Melan-a) c...
Embodiment 3
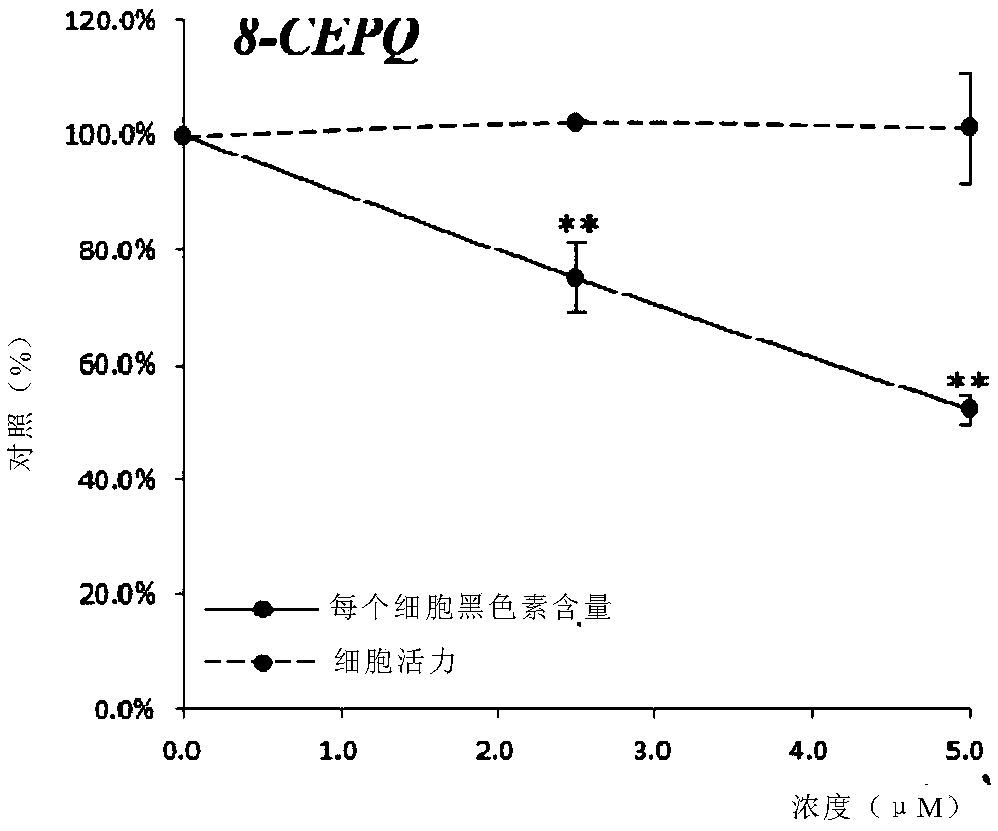
[0229] Example 3: Induction of melanosome autophagy by 6-CEPN and 8-CEPQ
[0230] Materials and methods
[0231] Cell Processing for Fluorescence Microscopy
[0232] Melanin A (Melan-a) cells (1.5×10 5 cells / well) were seeded on coverslips in 6-well plates. Cells were treated with (1) α-MSH (1 μM) alone for 24 hours, (2) α-MSH (1 μM) plus rapamycin (0.5 μM) for 24 hours, (3) α-MSH (1 μM) plus 6- CEPN (10 μM), or (3) α-MSH (1 μM) plus 8-CEPQ (5 μM) were treated for 72 hours. After the cells reached a confluency level of 50%-70%, the medium was carefully removed. Cells were stained by microscope dual detection reagent (autophagy detection kit, Abcam) at 37°C for 30 minutes. Stained cells were analyzed by widefield fluorescence microscopy (6Ox magnification). Set standard FITC filter and DAPI filter to image autophagy signal and nuclear signal, respectively.
[0233] siRNA transfection and western blot analysis
[0234] Melanin A (Melan-a) cells (3×10 5 cells / well) wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com