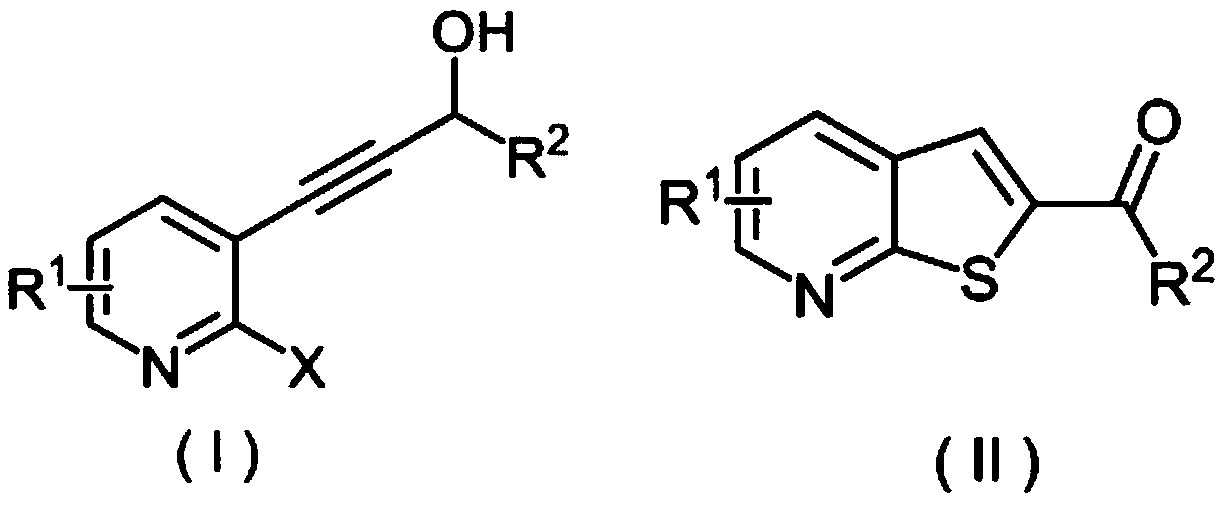
Method for synthesizing 2-carbonyl thienopyridine compound
A technology of phenopyridine and synthesis method, which is applied in the synthesis field of 2-carbonylthienopyridine compounds, can solve the problems of expensive starting materials, low atom economy and high synthesis cost, and achieves economical cost saving and product yield. The effect of high rate and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
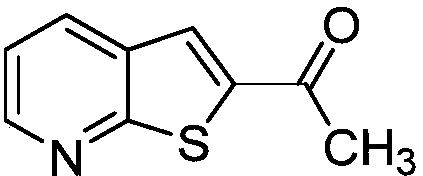
[0024] This example is the synthesis of 1-(thien[2,3-b]2-pyridyl)ethanone.
[0025]
[0026] (a) Take a reaction tube, add 33mmg (0.20mmol) of 2-fluoro-3-(3-hydroxy)but-1-ynepyridine, 64mg (0.40mmol) of potassium ethyl xanthate, and 13mmg of copper diacetylacetonate (0.05mmol), dimethyl sulfoxide 3mL, stirred at 100°C for 12 hours, after the reaction was completed, 10mL of ethyl acetate was added to quench the reaction, and 5mL of saturated brine was added to wash, the organic phase was separated, and the aqueous phase was washed with ethyl acetate Extracted 3 times (the amount of ethyl acetate each time was 5mL) and combined the organic phases, added anhydrous sodium sulfate to dry, removed the solvent by distillation under reduced pressure, and separated by column chromatography to obtain the target product 1-(thiophene[2,3-b ]2-pyridyl)ethanone, the yield is 93%.
[0027] (b) Take a reaction tube, add 33.0mmg (0.20mmol) of 2-fluoro-3-(3-hydroxy)but-1-ynepyridine, 64mmg ...
Embodiment 2
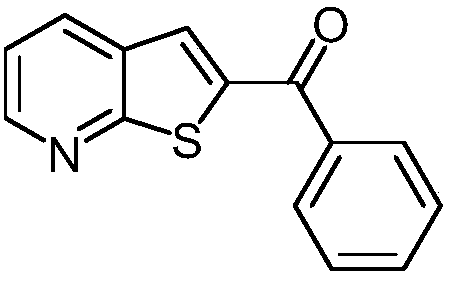
[0031] This example is the synthesis of phenyl(thien[2,3-b]2-pyridyl)methanone.
[0032]
[0033] Take a reaction tube, add 239mmg (1.05mmol) of 3-(2-fluoro-3-pyridyl)-1-phenylpropan-2-yn-1-ol and 320mmg (2.00mmol) of potassium ethyl xanthate respectively , 26 mmg (0.10 mmol) of copper diacetylacetonate, 3 mL of dimethyl sulfoxide, and stirred at 100° C. for 12 hours. At the end of the reaction, add 10 mL of ethyl acetate to quench the reaction, add 5 mL of saturated brine to wash, separate the organic phase, and then extract the aqueous phase with ethyl acetate for 3 times (the amount of ethyl acetate each time is 5 mL) to combine the organic phases, add anhydrous Dry over sodium sulfate, remove the solvent by distillation under reduced pressure, and separate by column chromatography to obtain the target product, phenyl(thiophene[2,3-b]2-pyridyl)methanone, with a yield of 88%.
[0034] The characterization data is 1 H NMR (400MHz, CDCl 3 )δ8.71(dd, J=4.5,1.4Hz,1H),8.16(...
Embodiment 3
[0036] This example is the synthesis of 1-(thien[2,3-b]2-pyridyl)propan-1-one.
[0037]
[0038] In the reaction tube, add 1-(2-fluoro-3-pyridyl)-pent-1-yn-3-ol 179mmg (1.00mmol), potassium ethyl xanthate 320mmg (2.00mmol), diacetylacetone 26 mmg (0.10 mmol) of copper and 3 mL of dimethyl sulfoxide were stirred and reacted at 100° C. for 12 hours. After the reaction was completed, 10 mL of ethyl acetate was added to quench the reaction, and 5 mL of saturated brine was added for washing. Dry over sodium sulfate, remove the solvent by distillation under reduced pressure, and separate by column chromatography to obtain the target product 1-(thien[2,3-b]2-pyridyl)propan-1-one with a yield of 93%.
[0039] The characterization data is 1 H NMR (400MHz, CDCl 3 )δ8.67(s,1H),8.16(d,J=8.1Hz,1H),7.88(s,1H),7.36(dd,J=8.0,4.6Hz,1H),3.04(q,J=7.3 Hz,2H),1.28(t,J=7.3Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ195.1, 163.3, 149.5, 143.2, 133.4, 132.7, 125.9, 120.2, 32.5, 8.3. GC-MS (EI, 70eV) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com