Escherichia coli expression system for production of oxalate oxidase, production method of oxalate oxidase and application thereof
A technology of oxalate oxidase and Escherichia coli, applied in biochemical equipment and methods, oxidoreductase, chemical instruments and methods, etc., can solve the problems of complex renaturation process, high cost, inactivity, etc., and achieve simple separation and purification process , The effect of low production cost and high specific vitality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
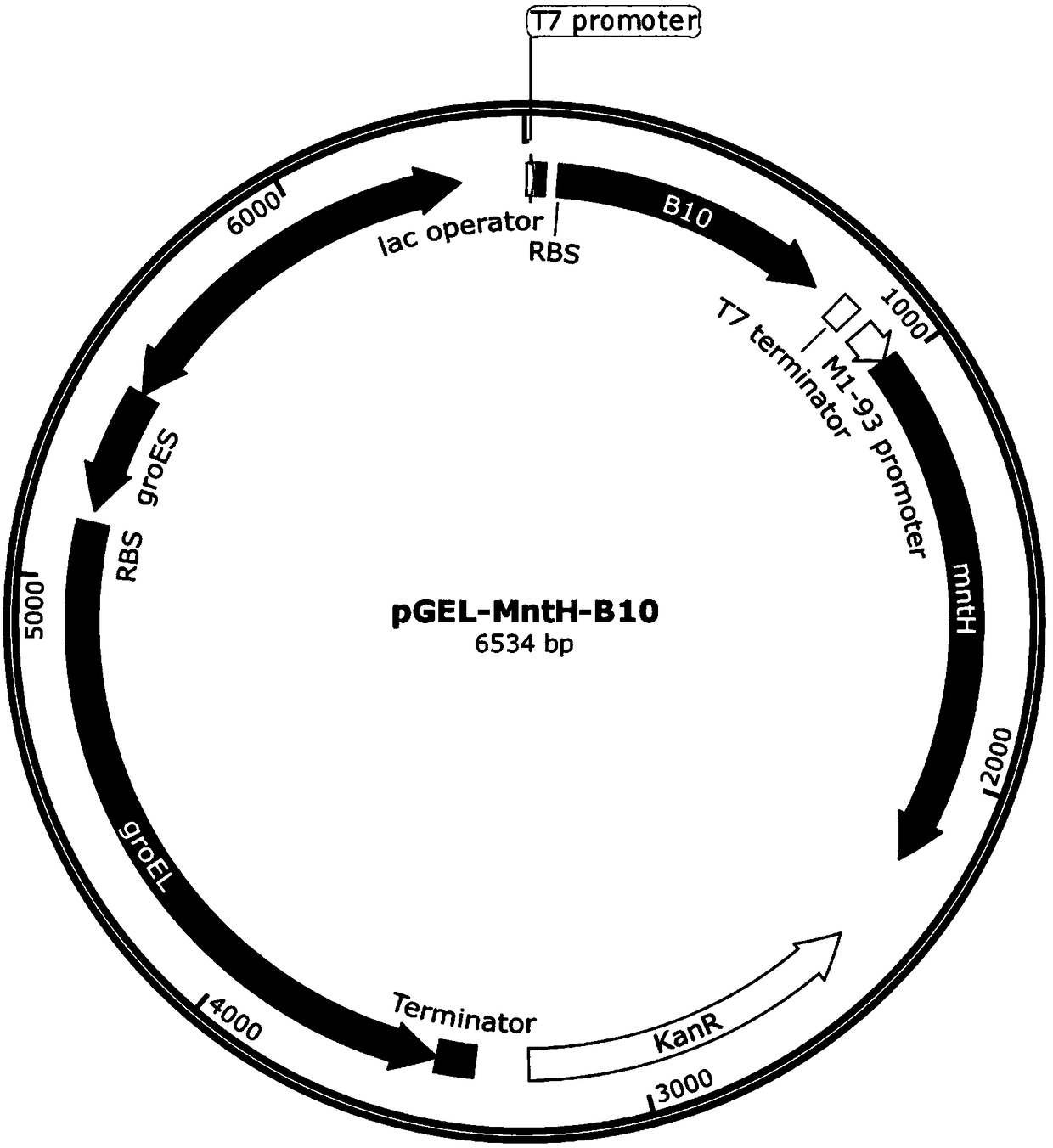
[0055] Example 1 Construction of expression vector pGEL-MntH-B10
[0056] Using the B10 gene (sequence shown in SEQ ID NO.1) synthesized from the whole gene as a template, design a primer pair F1 / R1, perform PCR amplification on the gene, and perform gel recovery and purification on the amplified product. The method refers to commercially available DNA According to the method in the instruction manual of the mini-purification kit, DNA fragment 1 (ie, the B10 gene fragment) was finally obtained. PCR system: 10×PCR Buffer 5μL, 2mMdNTP 5μL, 25mM MgSO 4 5 μL, 1.5 μL each of 10 μM primer F / R, template DNA 0.5 μL, KOD-Plus-Neo 1 μL, ddH2O 32.5 μL; PCR reaction conditions are as follows: 94 °C for 3 min, 30 cycles (98 °C for 10 s, 60 °C for 30 s, 68 °C 35s), 68°C for 5 minutes, and 4°C for 10 minutes; the PCR system in the description of the following vector construction is consistent with the above description, and will not be repeated below. The PCR reaction conditions are slight...
Embodiment 2
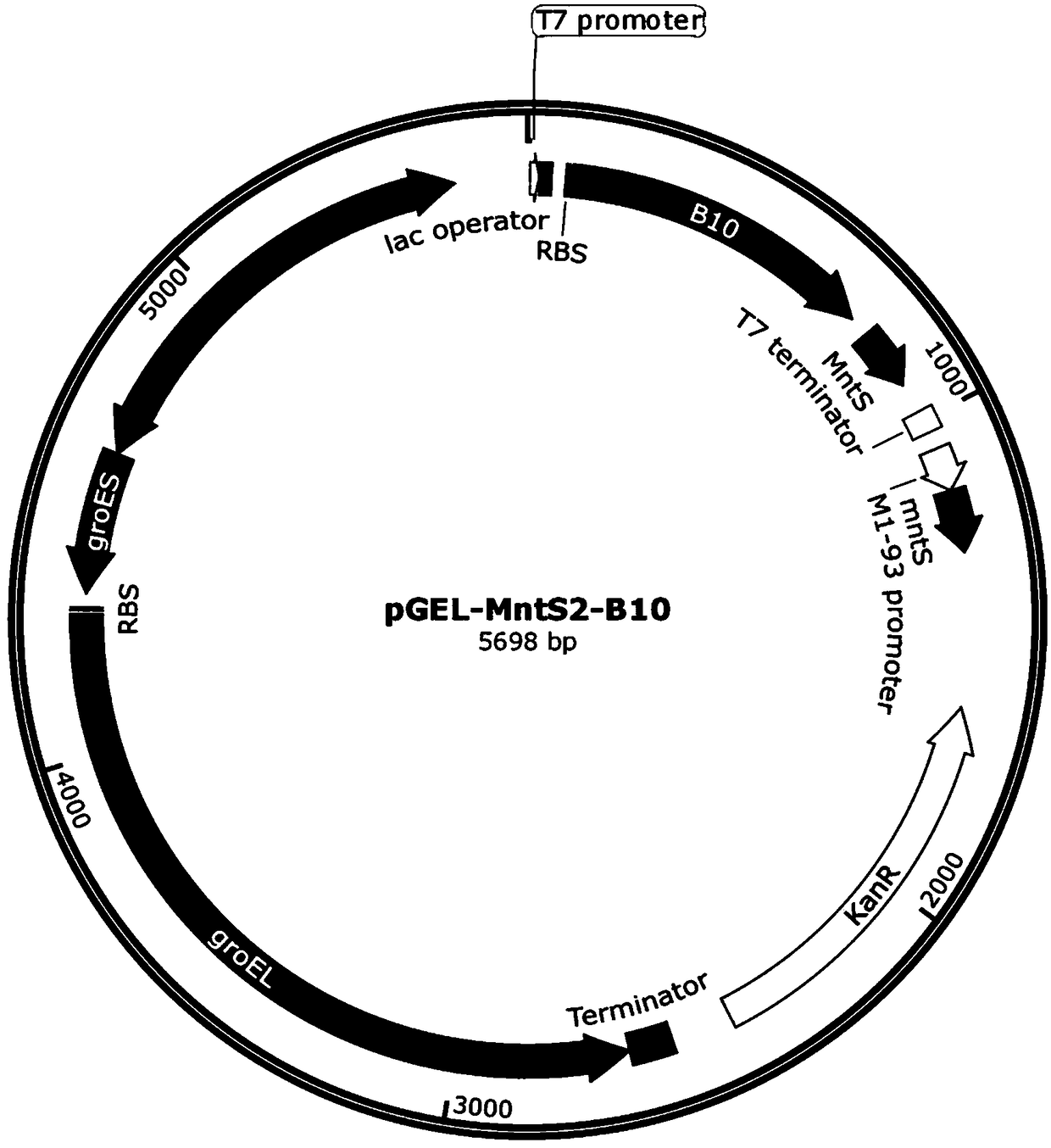
[0077] Example 2 Construction of expression vectors pGEL-MntS-B10 and pGEL-OxyR-B10
[0078]Using the genomic DNA of the Escherichia coli K12MG1655 strain as a template, the primer pair F9 / R9 was designed to amplify the DNA fragment of the MntS gene and the terminator (sequence shown in SEQ ID NO.8), and the amplified product was purified by the method of a DNA purification kit Finally, DNA fragment 9 was obtained; using the pGEL-MntH-B10 plasmid extracted by the plasmid mini-extraction kit as a template, primers were designed to amplify F8 / R10, and the product was purified to obtain DNA fragment 10; The above DNA fragments 9 and 10 were ligated, transformed into Escherichia coli DH5α, spread on the resistant LB solid medium plate containing 50 μg / ml karamycin for screening, verified positive clones by PCR and sequencing, and named the correct carrier after sequencing is pGEL-MntS-B10. The system and method of PCR amplification and seamless cloning used in this example are si...
Embodiment 3
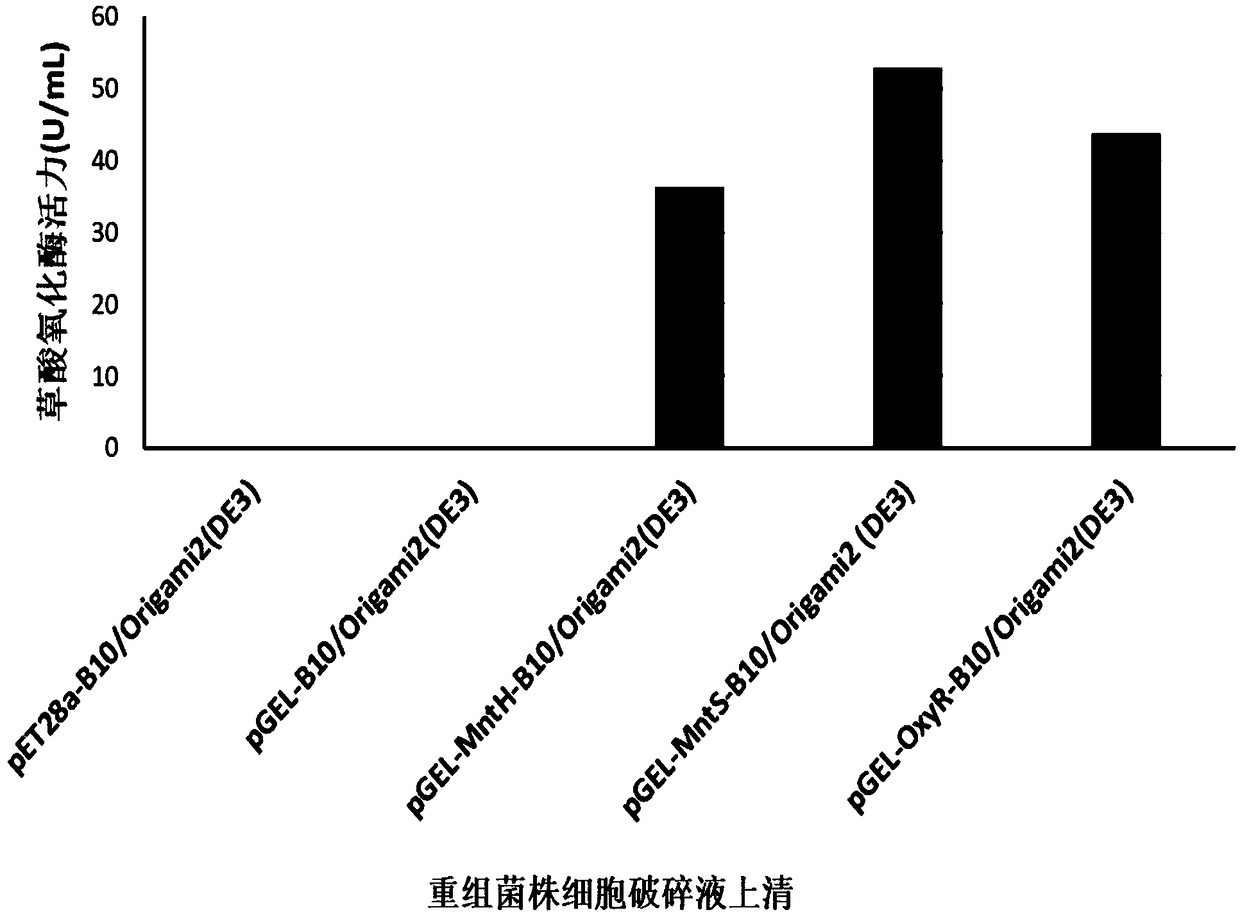
[0088] The expression test of embodiment 3 different manganese ion channel proteins
[0089] The plasmid DNA of pET28a-B10, pGEL-B10, pGEL-MntH-B10, pGEL-MntS-B10 and pGEL-OxyR-B10 was extracted by the method of plasmid mini-extraction kit, transformed into Origami2 (DE3) strain, and coated to contain 50 μg / ml karamycin-resistant LB solid medium plate, and positive clones were verified by PCR to obtain recombinant strains pET28a-B10 / Origami2(DE3), pGEL-B10 / Origami2(DE3), pGEL-MntH-B10 / Origami2 (DE3), pGEL-MntS-B10 / Origami2(DE3) and pGEL-OxyR-B10 / Origami2(DE3).
[0090] Inoculate the single clone on the resistant plate into LB liquid seed medium (yeast extract 0.5% (w / v), tryptone 1% (w / v), NaCl 1% (w / v), 20 μg / ml chloride Mycin, 50μg / ml kalamycin) to OD600=1.0-1.2, inoculated to JL medium (yeast extract 0.5% (w / v), tryptone 1.0 %(w / v), KH 2 PO 4 10mM, (NH 4 ) 2 SO 4 20mM, Mannitol 1.2% (w / v), Sodium Succinate 10mM, MgSO 4 0.15mM, initial pH 6.0-7.5), culture temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com