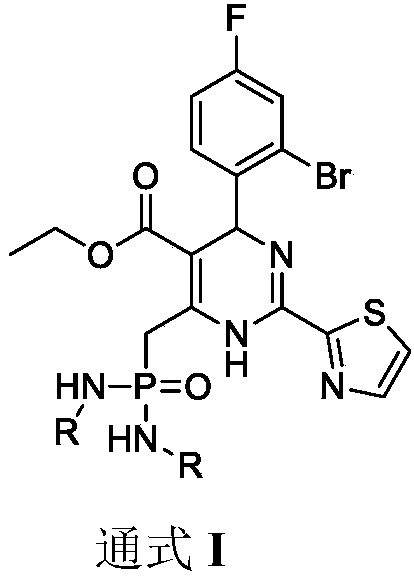
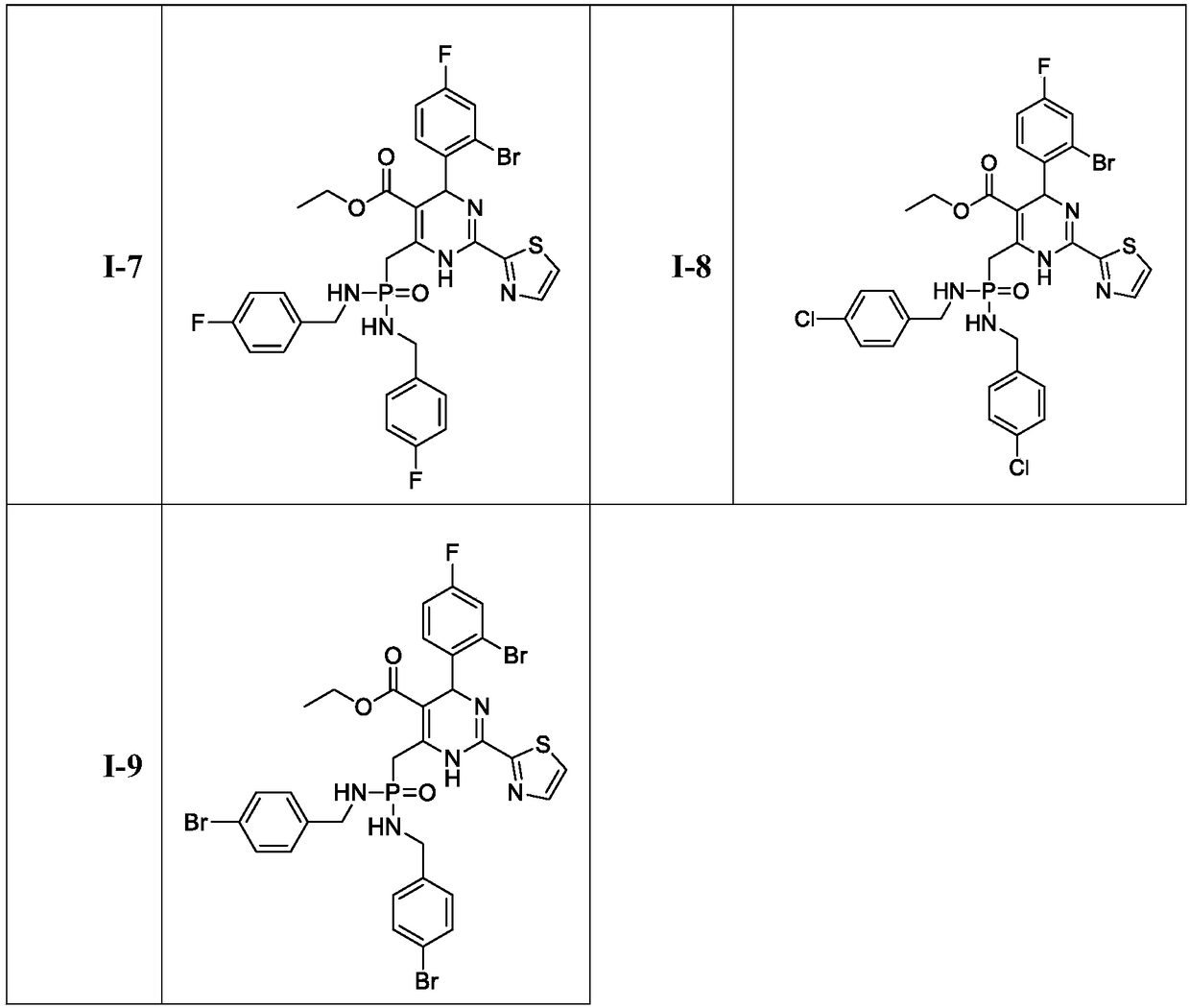
Dihydropyrimidine-phosphoramide derivative, and preparation method and application thereof
A dihydropyrimidine and phosphoramide technology, applied in the field of medicine, can solve problems such as poor water solubility, strong liver toxicity, and poor metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1. Preparation of Compound 2
[0043]Take a 100mL round-bottomed flask, dissolve 2-thiazolecarboxamidine hydrochloride (0.50g, 3.05mmol) in 50mL of absolute ethanol, and add 2-bromo-4-fluorobenzaldehyde (0.93g, 4.60mmol) successively at room temperature ), ethyl acetoacetate (600μL, 4.60mmol), sodium acetate (0.50g, 6.13mmol), reflux at 80°C for 6h; after the reaction, cool to room temperature, spin evaporate to remove absolute ethanol, add water (60mL), Extracted three times with ethyl acetate (25mL x 3), combined the organic phases, washed once with saturated brine (25mL), dried over anhydrous sodium sulfate; concentrated, dry-loaded, separated by flash preparative silica gel column, mixed dichloromethane-n-hexane Solvent recrystallization gave 0.75 g of a yellow solid with a yield of 58% and a melting point of 153-156°C.
[0044]
[0045] Compound 2 spectral data: 1 H NMR (400MHz, CDCl 3 )δ7.81(d, J=2.8Hz, 1H), 7.46(s, 1H), 7.38–7.28(m, 2H), 6.97(t, ...
Embodiment 2
[0046] Embodiment 2. Preparation of Compound 3
[0047] Take a 100mL round-bottomed flask, dissolve Intermediate 2 (0.50g, 1.17mmol) in 50mL carbon tetrachloride, slowly add NBS (0.22g, 1.24mmol), and reflux at 50°C for 2h; after the reaction, cool to room temperature , carbon tetrachloride was removed by rotary evaporation, water (50mL) was added, ethyl acetate extracted (20mL x 3), the organic phases were combined, washed once with saturated brine (25mL), dried over anhydrous sodium sulfate; concentrated, dry loading, Flash preparative chromatography on a silica gel column and recrystallization from a dichloromethane-n-hexane mixed solvent gave 0.35 g of a yellow solid with a yield of 59% and a melting point of 123-128°C.
[0048]
[0049] Spectral data of compound 3: 1 H NMR (400MHz, CDCl 3 )δ7.84(d,J=3.1Hz,1H),7.52(s,2H),7.44–7.35(m,1H),7.32(dd,J=8.1,2.6Hz,1H),7.02(t,J =8.0Hz,1H),6.09(s,1H),4.94(d,J=8.9Hz,1H),4.61(s,1H),4.09(d,J=7.0Hz,2H),1.16(t,J =7.1Hz, 3H); EI-MS...
Embodiment 3
[0050] Embodiment 3. Preparation of Compound 4
[0051] Take a 10mL round bottom flask, dissolve intermediate 3 (1.00g, 2.00mmol) in 3mL triethyl phosphite, heat the reaction at 160°C for 2h; after the reaction, cool to room temperature, remove triethyl phosphite by rotary evaporation, 5 mL of ethyl acetate was added to mix the sample, the sample was loaded by dry method, separated on a silica gel column by flash preparative chromatography, and recrystallized from a mixed solvent of dichloromethane-n-hexane to obtain 0.75 g of a yellow solid with a yield of 67%.
[0052]
[0053] Compound 4 spectral analysis data: 1 H NMR (400MHz, DMSO) δ9.63(s, 1H), 8.00(d, J=3.1Hz, 1H), 7.93(d, J=3.1Hz, 1H), 7.57(dd, J=8.5, 2.3Hz ,1H),7.46(dd,J=8.6,6.2Hz,1H),7.24(td,J=8.5,2.3Hz,1H),6.00(d,J=2.4Hz,1H),4.10–3.99(m, 6H), 3.95(q, J=7.1Hz, 2H), 1.15-1.25(m, 6H), 1.04(t, J=7.1Hz, 3H); EI-MS: 560.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com