Compound for positron imaging, intermediate and preparation method thereof, and imaging agent containing compound
A technology of compounds and intermediates, applied in the field of new PET molecular probes and their preparation, can solve problems such as difficulty in functioning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: 19 Preparation of F-BAR-3
[0095] In order to facilitate the study of the preparation method, first prepare the non-radioactive compound according to the following reaction formula.
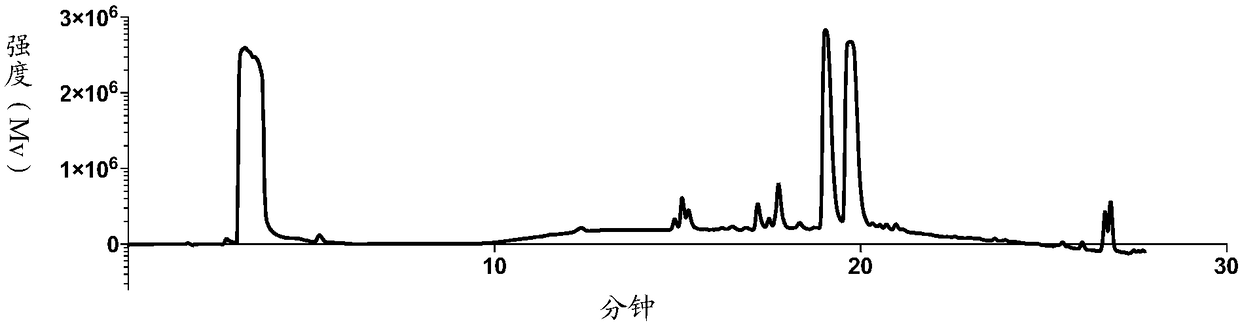
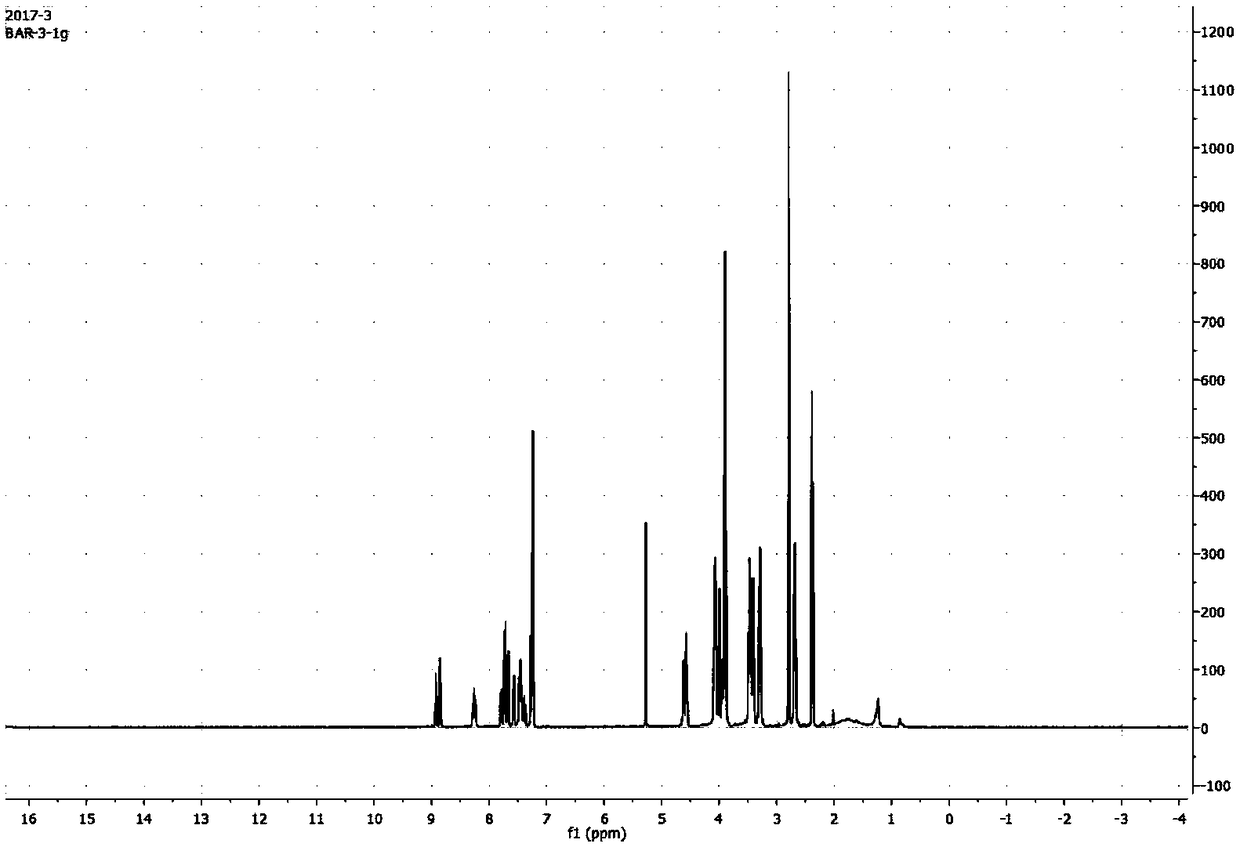
[0096] Mix 0.5 g of BAR-1 (i.e. GDC-0941, Selleck, USA) with 1.5 g of TsO-PEG 3 -OTs (Bailingwei) was dissolved in 50 mL of acetonitrile, 0.5 g of potassium carbonate was added, and reacted at 70° C. for 1 to 3 days. After the reaction was completed, the solvent was concentrated and removed, and the residue was purified by silica gel column chromatography to obtain 0.4 g of the product as a light yellow oil with a yield of 82%. The resulting product was mass spectrometered (m / z (ESI+), calculated value 799.25, measured value 800.25, mass spectrometer Expression CMS, Advion), 1H-NMR (400MHzDRX NMR Spectrometer, Bruker Corporation, see figure 2 ) and HPLC (Shimadzu LC-20A, chromatographic column Phenomenon Luna C18 (2), 250mm 4.6mm, see figure 1, wherein, the two peaks at 19...
Embodiment 2
[0100] Example 2: 18 Synthesis of F-BAR-3
[0101] In this example, the automatic synthesis equipment (TRACELAB FX Fn) of GE Company of the United States was used to carry out a fully automated one-step rapid synthesis.
[0102] By cyclotron (GE, minitrace) bombardment 3mL18 oxygen-water (H 2 18 O) generate 18 F-HF500mCi. to contain 18 The target water of F-HF passes through the QMA column (Waters, Accell Plus QMA) to 18 f - Ions are retained on the QMA cartridge. Then with 1.5 mL of acetonitrile aqueous solution containing 3 mg of potassium carbonate and 15 mg of phase transfer catalyst K2.2.2 (Sigma-Aldrich) (acetonitrile to water volume ratio 2:1) 18 f - Elute from the QMA column and enter the reaction flask, heat and nitrogen until the solvent in the reaction flask is evaporated to obtain dry anhydrous K 18 F (~450mCi).
[0103] Add 1.0 mL of anhydrous DMSO solution containing 2 mg of BAR-2 prepared in Example 1 into the reaction flask, react at 80°C for 30 minu...
Embodiment 3
[0106] Embodiment 3: 18 F-BAR-3 with 11 Comparison of Biodistribution and Metabolism of C-BAR-1
[0107] Investigate the prepared by the embodiment of the present invention 2 by PET imaging 18 Biodistribution and metabolism of F-BAR-3 in normal Kunming mice (female, 4 weeks old, purchased from Beijing Huafukang Biotechnology Co., Ltd.) 11 C-BAR-1 served as a control.
[0108] 11 Preparation of C-BAR-1:
[0109] According to the reaction formula, the automatic synthesis equipment (TRACELAB FX) of GE Company of the United States was adopted. C Pro) for fully automated one-step rapid synthesis 11 C-BAR-1.
[0110]
[0111] 500mCi generated externally by cyclotron (GE MINItrace) 11 CO 2 . 500mCi 11 CO 2 Transfer into the reactor with 100mL / min H 2 Hybrid, generated online 11 CH 4 500mCi. Subsequent reaction with sublimated iodine (iodine furnace temperature 100°C, about 20g of iodine particles) at a high temperature of 720°C to generate methyl iodide ( 11 CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com