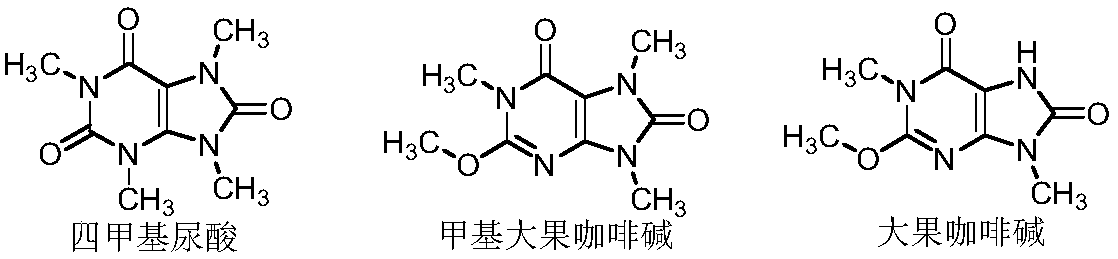
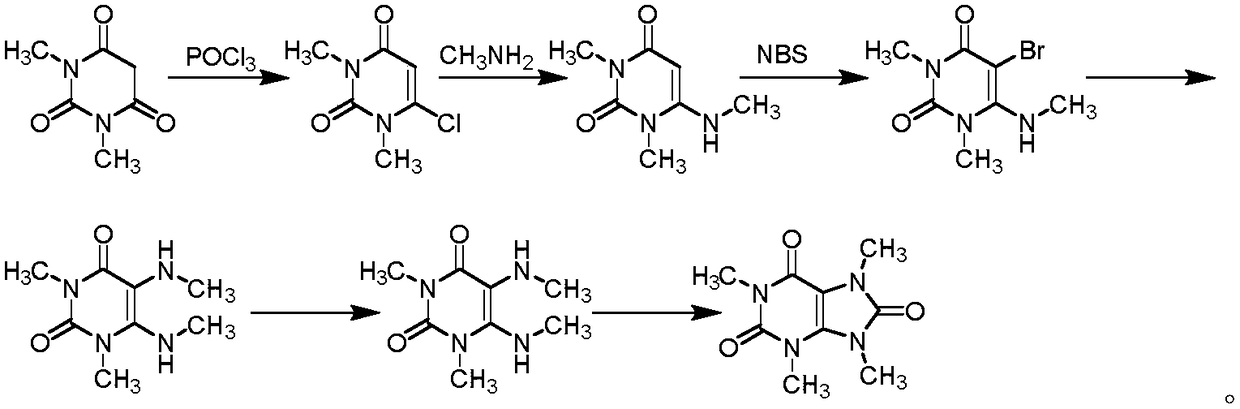
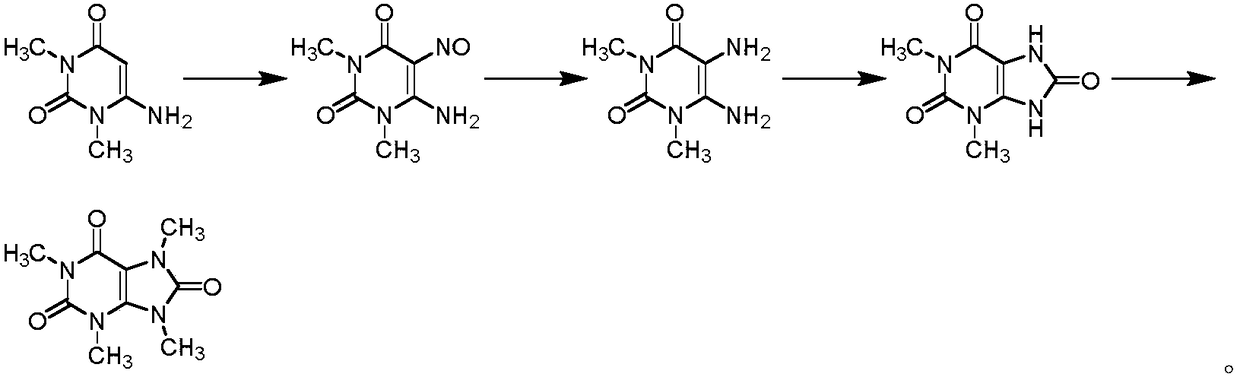
Preparation methods of three kinds of methyl uric acid compounds, intermediate and preparation method of the intermediate
A compound, methylation technology, applied in the fields of organic chemistry, digestive system, drug combination, etc., can solve the problems of no synthesis report and no industrial value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The preparation of embodiment 1 caffeine
[0070] (1) Preparation of 5,6-diamino-2-methoxy-3-methylpyrimidinyl-4-one
[0071]
[0072] 6-Amino-2-methoxy-3-methylpyrimidinyl-4-one (10g, 64.5mmol) was dissolved in tetrahydrofuran, and N-chlorosuccinimide (12.9g , 96.8mmol), after the addition was completed, the reaction was carried out at 20-30°C for 6 hours, and the reaction was basically complete. After concentration, add water and dichloromethane, separate the liquids, wash with sodium thiosulfate solution, sodium bicarbonate solution, and water successively, recrystallize the organic phase after concentration, and obtain off-white solid 6-amino-5-chloro-2- Methoxy-3-methylpyrimidinyl-4-one 10.4 g, yield 85%.
[0073] Suspend 6-amino-5-chloro-2-methoxy-3-methylpyrimidinyl-4-one (10.4g, 54.8mmol) in methanol, add ammonia water (35.8g, 548mmol), and react at 50-60°C After 12 hours, the reaction was substantially complete. Concentrate, add dichloromethane and water...
Embodiment 2
[0078] The preparation of embodiment 2 caffeine
[0079] (1) Preparation of 5,6-diamino-2-methoxy-3-methylpyrimidinyl-4-one
[0080]
[0081] 6-Amino-2-methoxy-3-methylpyrimidinyl-4-one (10 g, 64.5 mmol) was dissolved in tetrahydrofuran, and dibromohydantoin (13.8 g, 48.4 mmol) was added in portions at 20-30°C. After the addition is complete, react at 20-30°C for 6 hours, and the reaction is basically complete. After concentration, add water and dichloromethane, separate the liquids, wash with sodium thiosulfate solution, sodium bicarbonate solution, and water successively, recrystallize the organic phase after concentration, and obtain off-white solid 6-amino-5-bromo-2- Methoxy-3-methylpyrimidinyl-4-one 12.1 g, yield 80%.
[0082] 6-Amino-5-bromo-2-methoxy-3-methylpyrimidinyl-4-one (12.1g, 51.6mmol) was suspended in tetrahydrofuran, ammonia water (16.9g, 258mmol) was added, and sealed at 110-120°C After 1 hour of reaction, the reaction was almost complete. Concentrate,...
Embodiment 3
[0087] The preparation of embodiment 3 caffeine
[0088] (1) Preparation of 5,6-diamino-2-methoxy-3-methylpyrimidinyl-4-one
[0089]
[0090] 6-Amino-2-methoxy-3-methylpyrimidinyl-4-one (10g, 64.5mmol) was dissolved in toluene, and N-bromosuccinimide (51.6mmol ), react at 70-80°C for 1 hour after the addition, and the reaction is basically complete. After concentration, add water and dichloromethane, separate the liquids, wash with sodium thiosulfate solution, sodium bicarbonate solution, and water successively, recrystallize the organic phase after concentration, and obtain off-white solid 6-amino-5-chloro-2- Methoxy-3-methylpyrimidinyl-4-one 10.6 g, yield 87%.
[0091] Suspend 6-amino-5-bromo-2-methoxy-3-methylpyrimidinyl-4-one (10.4g, 54.8mmol) in toluene, add ammonia (54.8mmol), and react at 20-30°C for 24 hours , the reaction is almost complete. Concentrate, add dichloromethane and water solution, concentrate the organic phase, recrystallize with water, and dry to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com