A kind of highly active strigolactone derivative and its preparation and application
A plant and compound technology, applied in the field of plant hormones and chemical synthesis, can solve the problems of restricting large-scale promotion and use, many intermediate steps in synthesis, and high production cost, and achieves the effects of convenient synthesis, promotion of leaf senescence, and simple structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
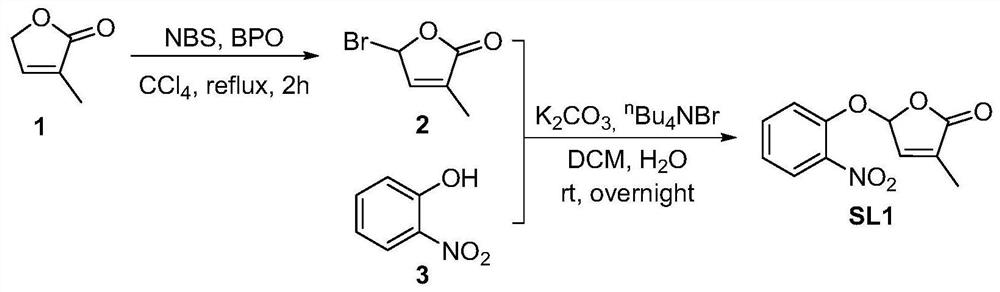
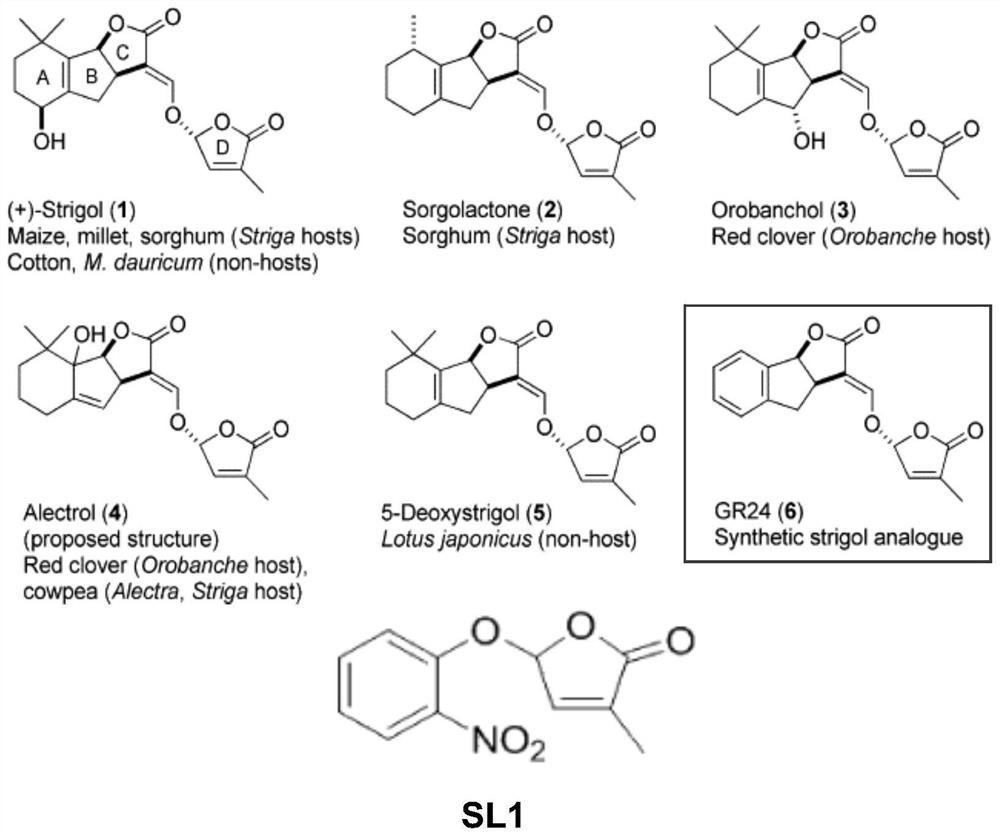
[0042] Embodiment 1, the preparation of strigolactone structural analogue SL1 shown in formula I
[0043] according to figure 1 The shown synthetic route diagram prepares the strigolactone structural analogue SL1 shown in formula I.
[0044] Starting lactone 1 (0.79 g, 8.05 mmol) was dissolved in CCl 4 (39mL), NBS (1.58g, 8.8mmol) and BPO (20mg, 0.083mmol) were added sequentially under stirring at room temperature, and heated to reflux for 2 hours. The reaction solution was filtered, and the filter cake was filtered with CCl 4 After washing, the filtrate was concentrated to give light yellow oil 2.
[0045] Bromide 2 was dissolved in DCM (32mL), and 2-nitrophenol (1.12g, 8.05mmol), tetrabutylammonium bromide (2.59g, 8.05mmol) and potassium carbonate (1.33g, 9.62mmol) were added successively under stirring at room temperature ) in aqueous solution (24mL), react overnight at room temperature. The reaction solution was diluted with EtOAc, and the organic phase was successive...
Embodiment 2
[0046] Example 2, SL1 promotes the interaction between Arabidopsis SMXL7 and AtD14
[0047] 1) Take 20ml 6×His-Flag-SMXL7 insect cell culture medium, centrifuge, discard the supernatant, and resuspend the cell pellet with 1mL buffer (50mM Tris-HCl pH7.0, 150mM NaCl, 0.5% Tween 20).
[0048] 2) Broken cells: liquid nitrogen-room temperature water was repeatedly frozen and thawed three times. After freezing and thawing, centrifuge (4°C, maximum speed for 10 minutes).
[0049] 3) After centrifugation, take the supernatant and incubate with Flag gel (Sigma) to combine, the condition is: 4°C, 1h.
[0050] 4) After the reaction, wash the unbound protein with buffer, then add 10 μg GST-D14 and 0, 1, 3, 5, 10 μM GR24 and SL1 for incubation, the condition is: 25°C, 1h.
[0051]5) After the reaction, discard the supernatant, and wash the unbound protein with buffer. Take 120uL 0.2mg / mlflag peptide to compete for elution, the conditions are: 4°C, 30min.
[0052] 6) After the reaction...
Embodiment 3
[0055] Example 3, SL1 inhibits the elongation of Arabidopsis hypocotyl
[0056] 1) Preparation of MS medium (0.6% agar) (1L formulation): 4.43g MS powder (Phytotechlab), 20g sucrose, 6g agar, pH 5.9-6.0. High-pressure steam sterilization at 121°C for 15 minutes.
[0057] 2) Sow the seeds of Col-0 and max2-3 (SALK_092836) in the MS medium supplemented with 0, 1, 3, 5, 10 μM of GR24 or SL1, and keep away from light at 4°C for three days.
[0058] 3) Take out the plate and put it in the plant room to grow for 7 days, the conditions are: low light and full light, 18-22°C.
[0059] 4) the seedlings are pulled out, placed on a 1% agar plate, scanned into a picture format, and the hypocotyl lengths of the seedlings under different treatments are measured with Digimizer software, and significant analysis is carried out with the ANOVA method of the SPSS software (results see Figure 5 ).
[0060] Depend on Figure 5 It can be seen that SL1 inhibits the elongation of the hypocotyl o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com