Preparation method and application of supported catalyst for methane dry reforming
A supported catalyst, methane dry reforming technology, applied in the direction of chemical instruments and methods, heterogeneous catalyst chemical elements, physical/chemical process catalysts, etc., can solve the problems of CO and H2 ratio deviation, deactivation, etc., to achieve high Stability, the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
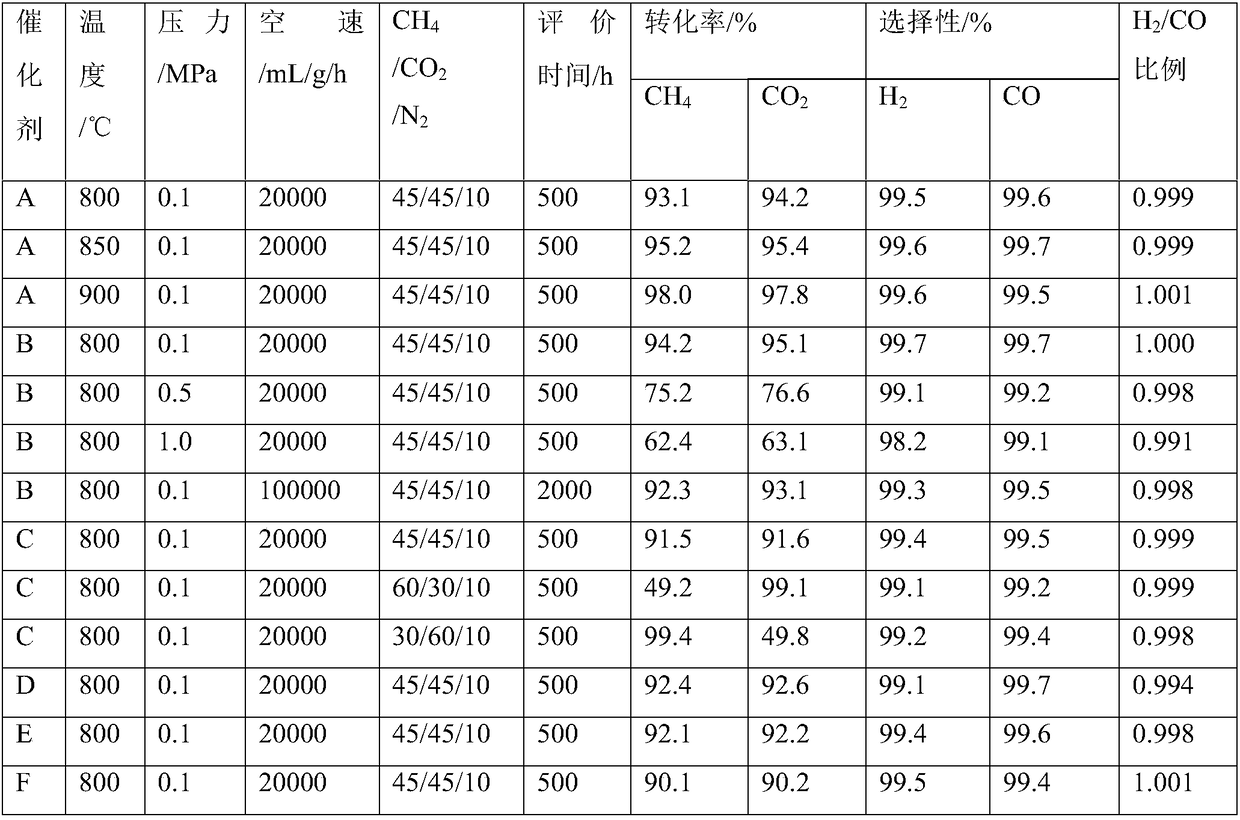
[0033] Weigh 5.82g of Ni(NO 3 ) 2 ·6H 2 O, 3.02g of glycine and 1.65g of NH 4 NO 3 It was dissolved in a certain amount of aqueous solution and stirred continuously at room temperature for 12 hours. Subsequently, 13.5 g of silicon dioxide was quickly added in an equal-volume preparation method and stirred evenly. It was dried under vacuum at 80°C, then dried in an oven at 120°C for 5 hours, and then calcined in a muffle furnace at 500°C for 5 hours to obtain catalyst powder. Finally, the 40-60 mesh particles were pressed, crushed and screened for activity evaluation, and the prepared catalyst was marked as catalyst A, wherein the mass fraction of NiO was 10.2%. The transmission electron microscope image (JEOL, JEM 2100) of the catalyst structure can be seen figure 1 , it can be seen that the particle size of the catalyst prepared by the combustion method is small, and the average particle size is only 5 nanometers.
Embodiment 2
[0035] Weigh 5.98g of Ni(NO 3 ) 2 ·6H 2 O, the ruthenium nitrate of 0.11g, the glycine of 3.14g and the NH of 1.78g 4 NO 3 It was dissolved in a certain amount of aqueous solution and stirred continuously at room temperature for 12 hours. Subsequently, 13.9 g of aluminum oxide was quickly added in an equal-volume preparation method, and stirred evenly. It was dried under vacuum at 80°C, then dried in an oven at 120°C for 5 hours, and then calcined in a muffle furnace at 500°C for 5 hours to obtain catalyst powder. Finally, the 40-60 mesh particles were pressed, crushed and screened for activity evaluation. The prepared catalyst was marked as catalyst B, in which the mass fraction of NiO was 10.6%, and the mass fraction of Ru was 0.2%.
Embodiment 3
[0037] Weigh 7.23g of palladium nitrate, 5.14g of urea and 2.12g of NH 4 NO 3 It was dissolved in a certain amount of aqueous solution and stirred continuously at room temperature for 12 hours. Subsequently, 14.2 g of aluminum oxide was quickly added in an equal-volume preparation method and stirred evenly. It was dried under vacuum at 100°C, then dried in an oven at 180°C for 12 hours, and then calcined in a muffle furnace at 700°C for 5 hours to obtain catalyst powder. Finally, the 40-60 mesh particles were pressed, crushed and screened for activity evaluation. The prepared catalyst was marked as Catalyst C, and the mass fraction of PdO was 9.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com