Small molecular blue-light emitting material and preparation method thereof
A technology of blue light materials and small molecules, applied in the fields of luminescent materials, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of unsatisfactory light color, attenuation of efficiency, insufficient efficiency, etc., to improve the luminous efficiency and reduce the interaction , increase the effect of steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
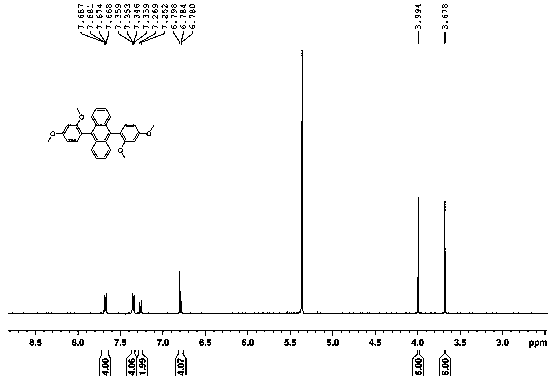
[0022] Example 1: Synthesis steps of 9,10-bis(2,4-dimethoxyphenyl)anthracene:
[0023] Add 9,10-dibromoanthracene and 2,4-dimethoxyphenylboronic acid into a round bottom flask at a molar ratio of 1:2, then add anhydrous sodium carbonate and catalyst [1,1'-bis(diphenyl Phosphinoyl) ferrocene] palladium dichloride 10% mol, use 1,4-dioxane and water volume ratio 4:1 as solvent, reflux at 110 ℃ overnight, evaporate the solvent after the reaction, water and di It was extracted with methyl chloride, dried over anhydrous sodium sulfate, and the mixture was separated through a silica gel column to obtain 9,10-bis(2,4-dimethoxyphenyl)anthracene as a white solid. The yield was 32%. 1H NMR (500 MHz, CD2Cl2): 3.68 (s, 6H, orthoMeO), 3.99 (s, 6H, paraMeO), 6.77-6.80 (m, 4H), 7.25-7.27(d, J=8.1 Hz, 2H), 7.33-7.37(m, 4H),7.66-7.69(m, 4H)
Embodiment 2
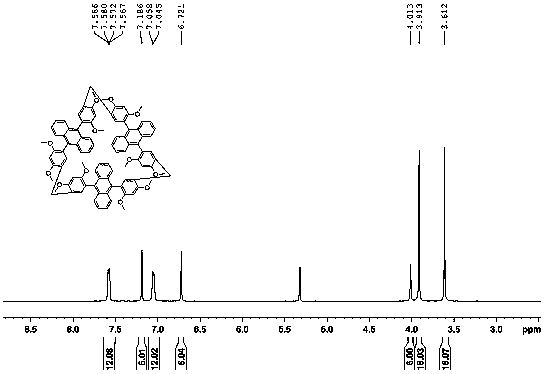
[0024] Example 2: Synthesis steps of cyclodimerization, cyclotrimerization and cyclotetramerization using 9,10-bis(2,4-dimethoxyphenyl)anthracene as monomer:
[0025] Add 9,10-bis(2,4-dimethoxyphenyl)anthracene and paraformaldehyde in a 50ml round bottom flask with a molar ratio of 1:5, use dichloromethane as solvent, add catalyst trifluoride after dissolution Boron ether, react at 25°C, spot plate observation. After the reaction was completed, it was quenched with a saturated sodium bicarbonate solution, washed with a saturated sodium chloride solution, dried over anhydrous sodium sulfate, and the resulting mixture was separated with a silica gel column to obtain the product cyclodimerization, cyclotrimerization, and cyclotetramerization yields respectively 7%, 3.5%, 0.6%.
[0026] Cyclodimer: 1H NMR (600 MHz, CD 2 Cl 2 ):3.58(s, 12H,), 3.89 (s, 12H), 4.06(m, 4H), 6.96(d, 6H), 7.31-7.35(m, 6H), 7.41-7.45(m, 4H), 7.65 -7.70(m, 8H)
[0027] Cyclotrimer: 1H NMR (500 MHz, CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com