Synthesizing method of ofloxacin and levofloxacin
A technology of levofloxacin and levofloxacin carboxylic acid, which is applied in the chemical field, can solve the problems of many impurities in related substances, unfriendly production environment, and poor reaction selectivity, and achieve the effects of good product quality, improved product appearance, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
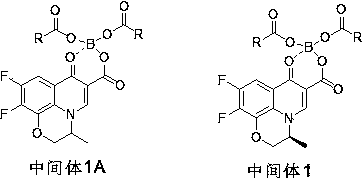
[0021] The preparation of embodiment 1 intermediate 1A
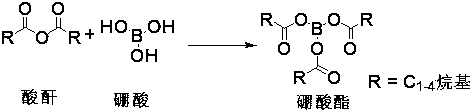
[0022] Throw 4.8g (77.6mmol) of boric acid and 33g (324mmol) of acetic anhydride into a 250ml flask, under nitrogen protection, stir and react at 75°C for 45min; raise the temperature of the system to 115°C and continue the reaction for 1h;
[0023] After the reaction of the above-mentioned borate ester system was completed, the temperature was lowered to 70°C, and 18g (64mmol) ofloxacin carboxylic acid was thrown into the flask; the temperature was raised to 90°C, and the reaction was carried out for 3h; the reaction was monitored by TLC (the developing agent was DCM: MeOH=20 :1). After the reaction, the system was poured into 200ml of 40% methanol-water mixed solvent (10°C), crystallized and stirred for 0.5h. Suction filtration, the filter cake was rinsed with 40ml of methanol. After drying to constant weight, 21 g of intermediate 1A was obtained with a yield of 80%.
Embodiment 2
[0024] The preparation of embodiment 2 intermediate 1A
[0025] Throw 4.8g (77.6mmol) of boric acid and 60g (461.5mmol) of propionic anhydride into a 250ml flask, under the protection of nitrogen, stir and react at 85°C for 30min; raise the temperature of the system to 115°C and continue the reaction for 2h;
[0026] After the reaction of the above-mentioned borate ester system was completed, the temperature was lowered to 80°C, and 16g (51.8mmol) ofloxacin ethyl carboxylate was thrown into the flask; the temperature was raised to reflux at 105°C, and the reaction was carried out for 1.5h; the reaction was monitored by TLC (developing agent was DCM : MeOH=20:1). After the reaction, pour the system into 200ml (0-10°C) 50% ethanol water, crystallize and stir for 0.5h. Suction filtration, the filter cake was rinsed with 40ml ethanol. After drying to constant weight, 20 g of intermediate 1A was obtained with a yield of 88%.
Embodiment 3
[0027] The preparation of embodiment 3 intermediate 1
[0028] Throw 4.8g (77.6mmol) of boric acid and 122.6g (776mmol) of butyric anhydride into a 250ml flask, under the protection of nitrogen, stir and react at 90°C for 1h; raise the temperature of the system to 120°C and continue the reaction for 2h;
[0029] After the reaction of the above-mentioned borate ester system was completed, the temperature was lowered to 75°C, and 13g (44mmol) of levofloxacin carboxylate methyl ester was thrown into the flask; the temperature was raised to 115°C, and the reaction was carried out for 2 hours; the reaction was monitored by TLC (the developing agent was DCM:MeOH=20:1 ). After the reaction, pour the system into 200ml (0-10°C) 30% benzyl alcohol water, crystallize and stir for 0.5h. Suction filtration, the filter cake was rinsed with 40ml ethanol. After drying to constant weight, 20 g of intermediate 1 was obtained, and the yield was 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com