Formulation for treatment of peripheral joints, spinal joints and/or extracellular matrix elements of connective tissue, method of manufacture and uses
A technology of exogenous matrix and preparation, applied in the field of pharmacy, can solve problems such as adverse effects, achieve large degradation, obvious therapeutic and anesthesia effects, and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Embodiment 1. Preparation of Formulation A
[0124] The composition of Formulation A is as follows:
[0125]
[0126] To manufacture 100 kg of Formulation A, the following steps were taken.
[0127](i) Add high-purity water in an amount of 71.69 kg to the first mixing tank, followed by glycerol in an amount of 3.0 kg and butanediol in an amount of 2 kg; the mixture is heated to 70° C. under constant stirring at 60 to 70 rpm.
[0128] (ii) Then add cetyl alcohol in an amount of 2.3 kg, glyceryl stearate in an amount of 3.8 kg, caprylic / capric triglyceride in an amount of 5.0 kg, caprylic acid in an amount of 1.0 kg to the second mixing tank cetyl esters, dimethicone in an amount of 1.0 kg, steareth-2 in an amount of 1.0 kg and steareth-20 in an amount of 0.66 kg; at 60 to 70 rpm The mixture was heated to 70°C with continuous stirring.
[0129] (iii) Add the mixture of (ii) to the first mixing tank during 5 minutes with constant stirring at 60 to 70 rpm, then allow ...
Embodiment 2
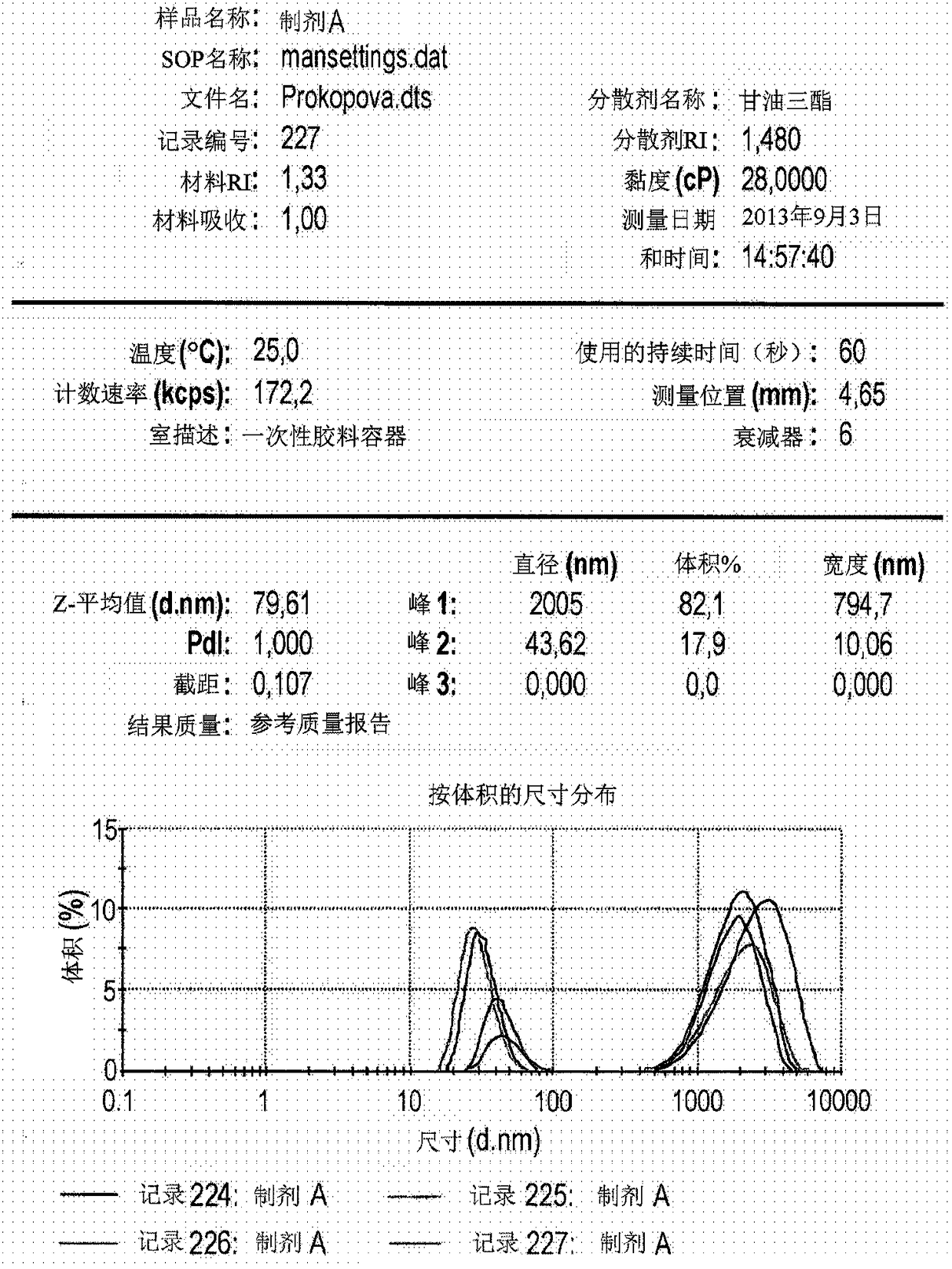
[0130] Example 2. Structural studies of Formulation A
[0131] The above characteristics of the structures formed in the dispersion (hydrodynamic diameter of the structure / size and its number by hydrodynamic diameter / size)( figure 1 ).
[0132] exist figure 1 , an automatically generated report showing the dependence of the relative volume occupied by the particles in the test sample on the particle size. For example, 17.9% of the total volume of the test sample occupied by the particles corresponds to particles with a hydrodynamic diameter of 15 nm to 80 nm, and an average hydrodynamic diameter of 43.62 nm, while 82.1% of the total volume of the test sample is accounted for by the hydrodynamic diameter Particles from 0.5 μm to 5 μm were occupied, and the mean hydrodynamic diameter of this subset was 2005 nm.
Embodiment 3
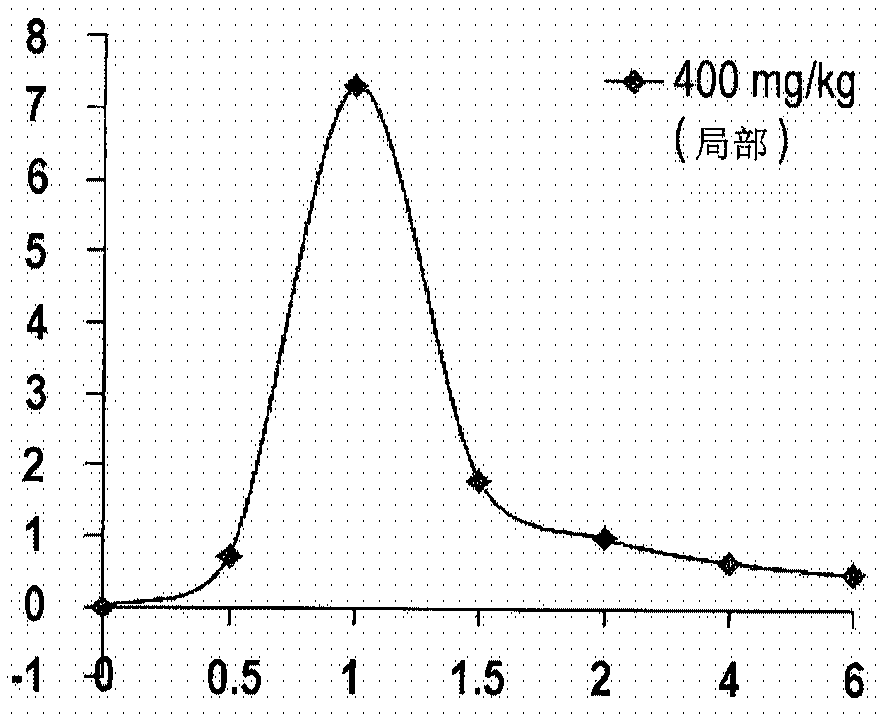
[0133] Example 3. Study of Glucosamine Bioavailability Using Formulation A
[0134] For the purpose of obtaining objective information on the bioavailability of glucosamine after dermal application, studies in rats have been performed.
[0135] A study comparing the relative bioavailability and permeability of glucosamine with oral and intramuscular administration of an aqueous glucosamine sulfate solution and topical formulation A cream has been performed in Sprague-Dawley rats.
[0136] The results presented below were obtained by measuring plasma glucosamine concentrations in rats. The study of 5 groups of rats with 9 rats in each group was performed according to the main study protocol. One group applied Formulation A cream topically, while another group administered glucosamine solution orally, and the other three groups - by injection at different concentrations of glucosamine.
[0137] Based on the analysis of the pharmacokinetic profile of glucosamine in rat plasma, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com